Chemistry Notes Form 4
Chemistry Notes Form 4 - Chemistry Online Revision - Chemistry Questions and Answers
At a school laboratory:
(i) An acid may be defined as a substance that turn litmus red.
(ii) A base may be defined as a substance that turn litmus blue.
Litmus is a lichen found mainly in West Africa.
It changes its colour depending on whether the solution it is in, is basic/alkaline or acidic.
It is thus able to identify/show whether
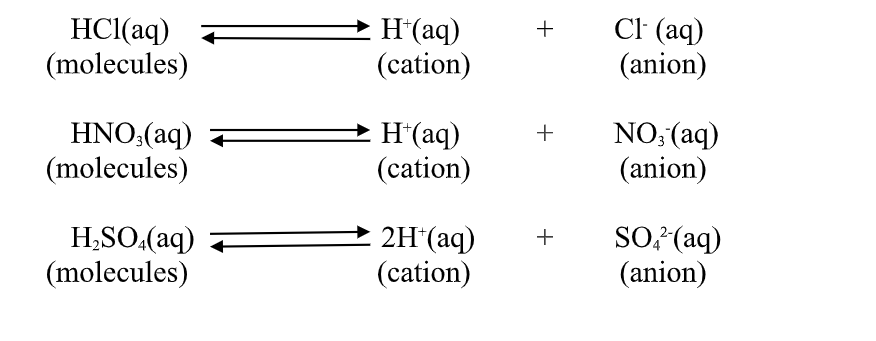
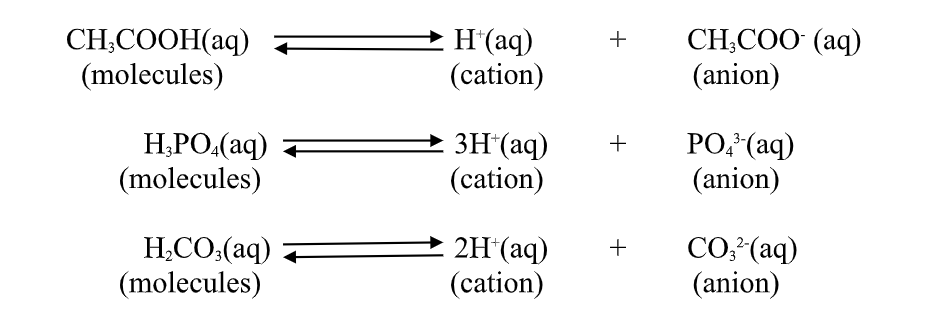
1. An acid is a substance that dissolves in water to form H-/H3O+ as the only positive ion/cation.
this is called the Arrhenius definition of an acid.
From this definition, an acid dissociate/ionize in water releasing H+ thus:
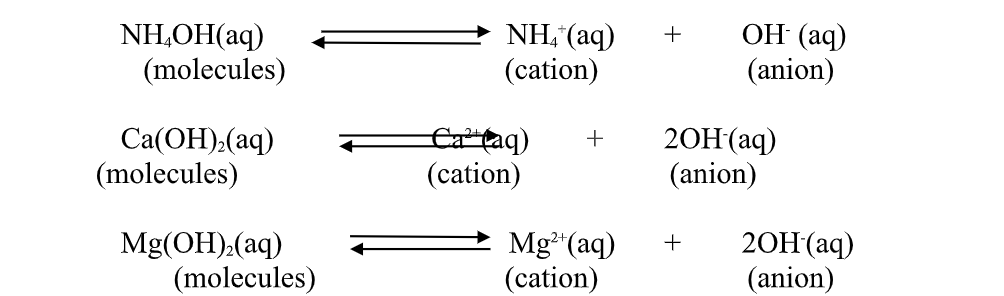
2.A base is a substance which dissolves in water to form OH- as the only negatively Charged ion/anion.
This is called Arrhenius definition of a base.
From this definition, a base dissociate/ionize in water releasing OH- thus:
3. An acid is a proton donor.
A base is a proton acceptor. this is called Bronsted-Lowry definition of acids and bases.
From this definition, an acid donates Hl+ .
Hl+ has no electrons and neutrons .It contains only a proton.
Examples
I. From the equation:
HCl(aq) + H2O(l) === H3O+(aq) + Cl-(aq)
(a)(i)For the forward reaction from left to right, H2O gains a proton to form HO+ and thus H2O is a proton acceptor .
It is a Bronsted-Lowry base
(ii) For the backward reaction from right to left, H3O+ donates a proton to form H3O and thus H3O+ is an ‘opposite’ proton donor.
It is a Bronsted-Lowry conjugate acid
(b)(i)For the forward reaction from left to right, HCl donates a proton to form Cl- and thus HCl is a proton donor .
It is a Bronsted-Lowry acid
(ii) For the backward reaction from right to left, Cl- gains a proton to form HCl and thus Cl- is an ‘opposite’ proton acceptor.
It is a Bronsted-Lowry conjugate base.
Every base /acid from Bronsted-Lowry definition thus must havea conjugate product/reactant.
II. From the equation:
HCl(aq) + NH3aq) === NH4+(aq) + Cl-(aq)
(a)(i) For the forward reaction from left to right, NH3 gains a proton to form NH4+ and thus NH3 is a proton acceptor .
It is a Bronsted-Lowry base
(ii) For the backward reaction from right to left, NH4+ donates a proton to form NH3 and thus NH4+ is an ‘opposite’ proton donor. It is a Bronsted-Lowry conjugate acid
(b)(i)For the forward reaction from left to right, HCl donates a proton to form Cl- and thus HCl is a proton donor .
It is a Bronsted-Lowry acid
(ii) For the backward reaction from right to left, Cl- gains a proton to form HCl and thus Cl- is an ‘opposite’ proton acceptor.
It is a Bronsted-Lowry conjugate base.
4. Acids and bases show acidic and alkaline properties/characteristics only in water but not in other solvents e.g.
(a) hydrogen chloride gas dissolves in water to form Hydrochloric acid Hydrochloric acid dissociates/ionizes in water to free H+(aq)/H3O+(aq) ions.
the free HO+(aq) / H+(aq) ions are responsible for:
(i) Turning blue litmus paper/solution red.
(ii) Show pH value 1/2/3/4/5/6
(iii) Are good electrolytes/conductors of electricity/undergo electrolysis.
(iv) React with metals to produce /evolve hydrogen gas and a salt. i.e.
Ionically:
-For a monovalent metal: 2M(s) + 2H+(aq) -> 2M+(aq) + H2(g)
-For a divalent metal: M(s) + 2H+(aq) -> M2+(aq) + H2(g)
-For a trivalent metal: 2M(s) + 6H+(aq) -> 2M3+(aq) + 3H2(g)
Examples:
-For a monovalent metal: 2Na(s) + 2H+(aq) -> 2Na+(aq) + H2(g)
-For a divalent metal: Ca(s) + 2H+(aq) -> Ca2+(aq) + H2(g)
-For a trivalent metal: 2Al(s) + 6H+(aq) -> 2Al3+(aq) + 3H2(g)
(v) React with metal carbonates and hydrogen carbonates to produce /evolve carbon(IV)oxide gas ,water and a salt. i.e.
Ionically:
-For a monovalent metal: M2CO3(s)+ 2H+(aq) -> 2M+(aq) + H2O (l)+ CO2(g)
MHCO3(s)+ H+(aq) -> M+(aq) + H2O (l)+ CO2(g)
-For a divalent metal: MCO3(s)+ 2H+(aq) -> M2+(aq) + H2O (l)+ CO2(g)
M(HCO3) 2(aq)+2H+(aq) ->M2+(aq)+2H2O(l)+2CO2(g)
Examples:
-For a monovalent metal: K2CO3(s)+ 2H+(aq) -> 2K+(aq) + H2O (l)+ CO2(g)
NH4HCO3(s)+ H+(aq) -> NH4+(aq) + H2O (l)+ CO2(g)
-For a divalent metal: ZnCO3(s)+ 2H+(aq) -> Zn2+(aq) + H2O (l)+ CO2(g)
Mg(HCO3) 2(aq)+2H+(aq) ->Mg2+(aq)+2H2O(l)+2CO2(g)
(vi) Neutralize metal oxides/Hydroxides to salt and water only. i.e.
Ionically:
-For a monovalent metal: M2O(s) + 2H+(aq) -> 2M+(aq) + H2O (l)
MOH(aq) + H+(aq) -> M+(aq) + H2O (l)
-For a divalent metal: MO(s) + 2H+(aq) -> M2+(aq) + H2O (l)
M(OH) 2(s) + 2H+(aq) -> M2+(aq) + 2H2O(l)
-For a trivalent metal: M2O3(s) + 6H+(aq) -> 2M3+(aq) + 3H2O (l)
M(OH) 3(s) + 3H+(aq) -> M3+(aq) + 3H2O(l)
Examples:
-For a monovalent metal: K2O(s) + 2H+(aq) -> 2K+(aq) + H2O (l)
NH4OH(aq) + H+(aq) -> NH4+(aq) + H2O (l)
-For a divalent metal: ZnO (s) + 2H+(aq) -> Zn2+(aq) + H2O (l)
Pb(OH) 2(s) + 2H+(aq) -> Pb2+(aq) + 2H2O(l)
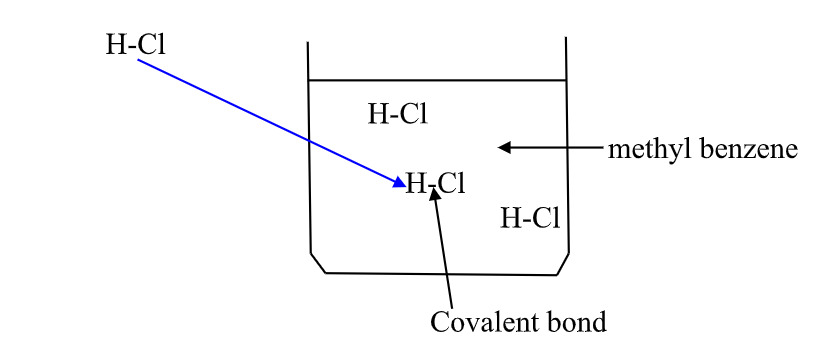
(b) hydrogen chloride gas dissolves in methylbenzene /benzene but does not dissociate /ionize into free ions.
It exists in molecular state showing none of the above properties.
(c) Ammonia gas dissolves in water to form aqueous ammonia which dissociate/ionize to free NH4+ (aq) and OH-(aq) ions.
thisdissociation/ionization makes aqueous ammonia to:
(i) Turn litmus paper/solution blue.
(ii) havepH 8/9/10/11
(iii) Be a good electrical conductor
(iv) React with acids to form ammonium salt and water only.
NH4OH(aq) + HCl(aq) -> NH4Cl(aq) + H2O(l)
(d) Ammonia gas dissolves in methylbenzene/benzene /kerosene but does not dissociate into free ions therefore existing as molecules
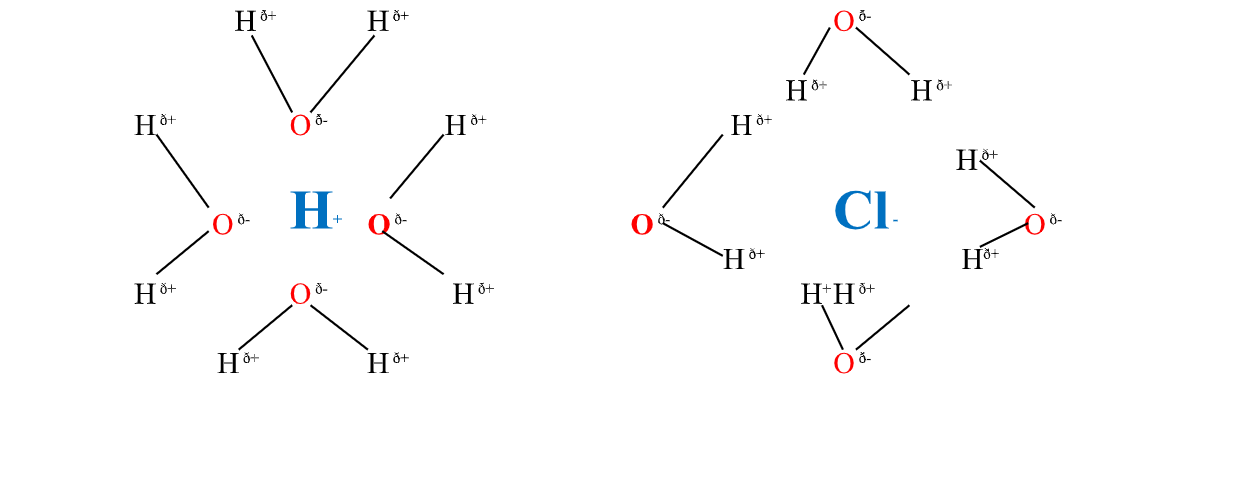
6. Solvents are either polar or non- Polar.
A polar solvent is one which dissolves ionic compounds and other polar solvents.
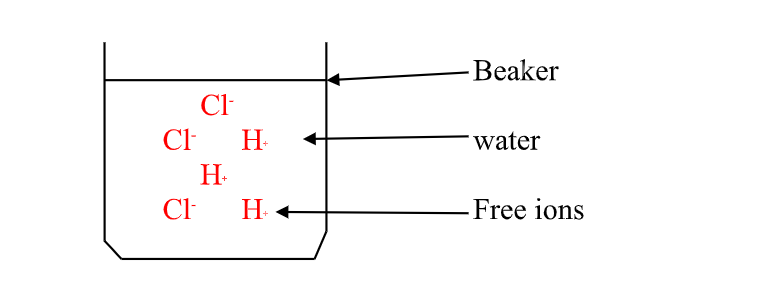
Water is polar solvent that dissolves ionic and polar substance by surrounding the free ions as below:
Oxygen atom is partially negative and two hydrogen atoms which are partially positive.
they surround the free H+ and Cl-ions.
A non polar solvent is one which dissolved non- Polar substances and covalent compounds.
If a polar ionic compound is dissolved in non- Polar solvent ,it does not ionize/dissociate into free ions as below:
(a) A strong acid/base is one which is fully/wHolly/completely dissociated / ionized into many free H+ /OH- ions i.e.
I. Strong acids exists more as free H+ ions than molecules. e.g.
I. Weak acids exists more as molecules than as free H+ ions. e.g.
An acid/base/alkali with more acid/base/alkali in a decimeter(litre) of solution is said to be concentrated wHile that with less is said to be dilute.
9. (a) (i)Strong acids havepH 1/2/3 while weak acids haveHigh pH 4/5/6.
(ii)a neutral solution havepH 7
(iii)strong alkalis/bases havepH 12/13/14 while weak bases/alkalis havepH 11/10 /9 / 8.
(b) pH is a measure of H+(aq) concentration in a solution.
the higher the H+(aq)ions concentration ;
- The higher the acidity
- The lower the pH
- The lower the concentration of OH-(aq)
- The lower the alkalinity
At pH 7 , a solution has equal concentration of H+(aq) and OH-(aq).
Beyond pH 7,the concentration of the OH-(aq) increases as the H+(aq) ions decreases.
10.(a) when acids /bases dissolve in water, the ions present in the solution conduct electricity.
the more the dissociation the higher the yield of ions and the greater the electrical conductivity of the solution.
A compound that conducts electricity in an electrolyte and thus a compound showing High electrical conductivity is a strong electrolyte wHile a compound showing low electrical conductivity is a weak electrolyte.
(b) Practically, a bright light on a bulb ,a High voltage reading from a voltmeter High ammeter reading from an ammeter, a big deflection on a galvanometer is an indicator of strong electrolyte(acid/base) and the opposite for weak electrolytes(acids/base)
11. Some compounds exhibit/show both properties of acids and bases/alkalis.
A substance that reacts with both acids and bases is said to be amphotellic.
the examples below show the amphotellic properties of:
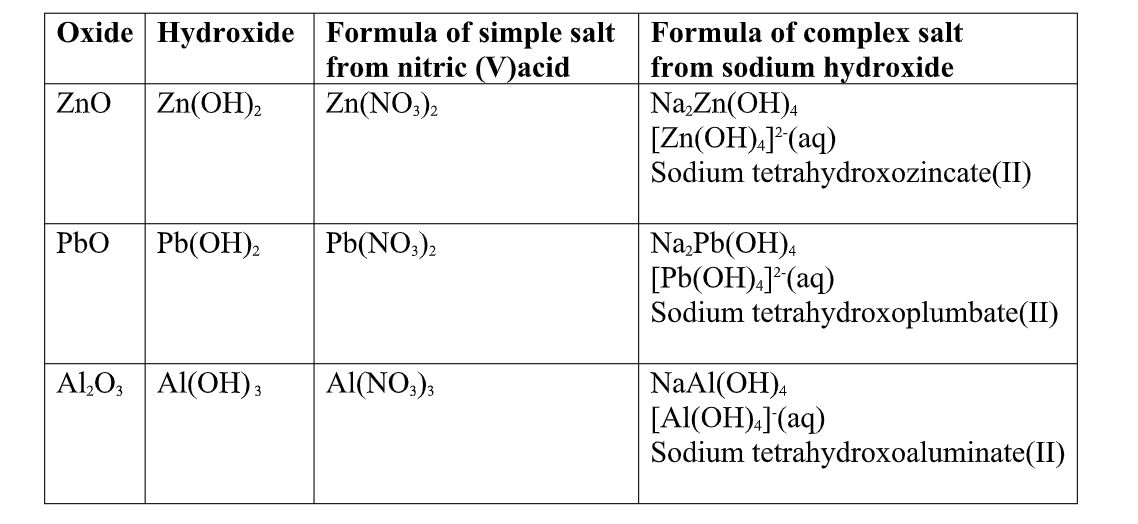
(a) Zinc (II)oxide(ZnO) and Zinc Hydroxide(Zn(OH)2)
(i)when ½ spatula full of Zinc(II)oxide is placed in a boiling tube containing 10cm3 of either 2M nitric(V)acid or 2M sodium Hydroxide Hydroxide solution, it dissolves on both the acid and the alkali/base to form a colourless solution. i.e.
(i) when reacting with nitric(V)acid, the oxide shows basic properties by reacting with an acid to form a simple salt and water only.
Basic oxide + Acid -> salt + water
Examples:
chemical equation
ZnO(s) + 2HNO3(aq) -> Zn(NO3) 2 (aq) + H2O(l)
ZnO(s) + 2HCl(aq) -> ZnCl2 (aq) + H2O(l)
ZnO(s) + H2SO4(aq) -> ZnSO4 (aq) + H2O(l)
Ionic equation
ZnO(s) + 2H+ (aq) -> Zn 2+ (aq) + H2O(l)
(ii) when reacting with sodium Hydroxide, the oxide shows acidic properties by reacting with a base to form a complex salt.
Basic oxide + Base/alkali + Water -> Complex salt
Examples:
chemical equation
1.when Zinc oxide is reacted with sodium Hydroxide the complex salt is sodium tetraHydroxozincate(II) complex salt.
ZnO(s) + 2NaOH(aq) + H2O(l) -> Na2Zn(OH) 4(aq)
2.when Zinc oxide is reacted with potassium Hydroxide the complex salt is potassium tetraHydroxozincate(II) complex salt.
ZnO(s) + 2KOH(aq) + H2O(l) -> K2Zn(OH) 4(aq)
Ionic equation
ZnO(s) + 2OH-(aq) + H2O(l) -> 2[Zn(OH) 4]2- (aq)
(ii)when Zinc(II)Hydroxide is placed in a boiling tube containing 10cm3 of either 2M nitric(V)acid or 2M sodium Hydroxide Hydroxide solution, it dissolves on both the acid and the alkali/base to form a colourless solution. i.e.
(i) when reacting with nitric(V)acid, the Hydroxide shows basic properties. It reacts with an acid to form a simple salt and water only.
Basic Hydroxide + Acid -> salt + water
Examples:
chemical equation
Zn(OH)2 (s) + 2HNO3(aq) -> Zn(NO3) 2 (aq) + 2H2O(l)
Zn(OH) 2 (s) + 2HCl(aq) -> ZnCl2 (aq) + 2H2O(l)
Zn(OH) 2 (s) + H2SO4(aq) -> ZnSO4 (aq) + 2H2O(l)
Ionic equation
Zn(OH) 2 (s) + 2H+ (aq) -> Zn 2+ (aq) + 2H2O(l)
(ii) when reacting with sodium Hydroxide, the Hydroxide shows acidic properties by reacting with a base to form a complex salt.
Basic Hydroxide + Base/alkali -> Complex salt
Examples:
chemical equation
1.when Zinc Hydroxide is reacted with sodium Hydroxide the complex salt is sodium tetraHydroxozincate(II) complex salt.
Zn(OH)2 (s) + 2NaOH(aq) -> Na2Zn(OH)4(aq)
2.when Zinc Hydroxide is reacted with potassium Hydroxide the complex salt is potassium tetraHydroxozincate(II) complex salt.
Zn(OH)2 (s) + 2KOH(aq) -> K2Zn(OH)4(aq)
Ionic equation
Zn(OH)2(s) + 2OH-(aq) -> 2[Zn(OH)4]2- (aq)
(b) Lead (II)oxide(PbO) and Lead(II) Hydroxide (Pb(OH)2)
(i)when ½ spatula full of Lead(II)oxide is placed in a boiling tube containing 10cm3 of either 2M nitric(V)acid or 2M sodium Hydroxide Hydroxide solution, it dissolves on both the acid and the alkali/base to form a colourless solution. i.e.
(i) when reacting with nitric(V)acid, the oxide shows basic properties by reacting with an acid to form a simple salt and water only. All other Lead salts are insoluble.
chemical equation
PbO(s) + 2HNO3(aq) -> Pb(NO3)2 (aq) + H2O(l)
Ionic equation
PbO(s) + 2H+ (aq) -> Pb 2+ (aq) + H2O(l)
(ii) when reacting with sodium Hydroxide, the oxide shows acidic properties by reacting with a base to form a complex salt.
chemical equation
1.when Lead(II) oxide is reacted with sodium Hydroxide the complex salt is sodium tetraHydroxoplumbate(II) complex salt.
PbO(s) + 2NaOH(aq) + H2O(l) -> Na2Pb(OH) 4(aq)
2.when Lead(II) oxide is reacted with potassium Hydroxide the complex salt is potassium tetraHydroxoplumbate(II) complex salt.
PbO(s) + 2KOH(aq) + H2O(l) -> K2Pb(OH) 4(aq)
Ionic equation
PbO(s) + 2OH-(aq) + H2O(l) -> 2[Pb(OH) 4]2- (aq)
(ii)when Lead(II)Hydroxide is placed in a boiling tube containing 10cm3 of either 2M nitric(V)acid or 2M sodium Hydroxide Hydroxide solution, it dissolves on both the acid and the alkali/base to form a colourless solution. i.e.
(i) when reacting with nitric(V)acid, the Hydroxide shows basic properties. It reacts with the acid to form a simple salt and water only.
chemical equation
Pb(OH)2(s) + 2HNO3(aq) -> Pb(NO3)2 (aq) + 2H2O(l)
Ionic equation
Pb(OH)2 (s) + 2H+ (aq) -> Pb 2+ (aq) + 2H2O(l)
(ii) when reacting with sodium Hydroxide, the Hydroxide shows acidic properties. It reacts with a base to form a complex salt.
chemical equation
1.when Lead(II) Hydroxide is reacted with sodium Hydroxide the complex salt is sodium tetraHydroxoplumbate(II) complex salt.
Pb(OH) 2 (s) + 2NaOH(aq) -> Na2Pb(OH) 4(aq)
2.when Lead(II) Hydroxide is reacted with potassium Hydroxide the complex salt is potassium tetraHydroxoplumbate(II) complex salt.
Pb(OH)2 (s) + 2KOH(aq) -> K2Pb(OH)4(aq)
Ionic equation
Pb(OH)2 (s) + 2OH-(aq) -> 2[Pb(OH) 4]2- (aq)
(c)Aluminium(III)oxide(Al2O3) and Aluminium(III)Hydroxide(Al(OH)3)
(i)when ½ spatula full of Aluminium(III)oxide is placed in a boiling tube containing 10cm3 of either 2M nitric(V)acid or 2M sodium Hydroxide Hydroxide solution, it dissolves on both the acid and the alkali/base to form a colourless solution. i.e.
(i) when reacting with nitric(V)acid, the oxide shows basic properties by reacting with an acid to form a simple salt and water only.
chemical equation
Al2O3 (s) + 6HNO3(aq) -> Al(NO3)3 (aq) + 3H2O(l)
Al2O3 (s) + 6HCl(aq) -> AlCl3 (aq) + 3H2O(l)
Al2O3 (s) + 3H2SO4(aq) -> Al2(SO4)3 (aq) + 3H2O(l)
Ionic equation
Al2O3 (s) + 3H+ (aq) -> Al 3+ (aq) + 3H2O(l)
(ii) when reacting with sodium Hydroxide, the oxide shows acidic properties by reacting with a base to form a complex salt.
chemical equation
1.when Aluminium(III) oxide is reacted with sodium Hydroxide the complex salt is sodium tetrahydroxoaluminate(III) complex salt.
Al2O3 (s) + 2NaOH(aq) + 3H2O(l) -> 2NaAl(OH) 4(aq)
2.when Aluminium(III) oxide is reacted with potassium Hydroxide the complex salt is potassium tetrahydroxoaluminate(II) complex salt.
Al2O3 (s) + 2KOH(aq) + 3H2O(l) -> 2NaAl(OH) 4(aq)
Ionic equation
Al2O3 (s) + 2OH-(aq) + 3H2O(l) -> 2[Al(OH) 4]- (aq)
(ii)when Aluminium(III)Hydroxide is placed in a boiling tube containing 10cm3 of either 2M nitric(V)acid or 2M sodium Hydroxide Hydroxide solution, it dissolves on both the acid and the alkali/base to form a colourless solution. i.e.
(i) when reacting with nitric(V)acid, the Hydroxide shows basic properties. It reacts with the acid to form a simple salt and water only.
chemical equation
Al(OH) 3 (s) + 3HNO3(aq) -> Al(NO3)3 (aq) + 3H2O(l)
Al(OH) 3 (s) + 3HCl(aq) -> AlCl3 (aq) + 3H2O(l)
2Al(OH)3 (s) + 3H2SO4(aq) -> Al2(SO4) 3 (aq) + 3H2O(l)
Ionic equation
Al(OH) 3 (s) + 3H+ (aq) -> Al 3+ (aq) + 3H2O(l)
(ii) when reacting with sodium Hydroxide, the Hydroxide shows acidic properties. It reacts with a base to form a complex salt.
chemical equation
1.when aluminium(III) Hydroxide is reacted with sodium Hydroxide the complex salt is sodium tetrahydroxoaluminate(III) complex salt.
Al(OH) 3 (s) + NaOH(aq) -> NaAl(OH)4(aq)
2.when aluminium(III) Hydroxide is reacted with potassium Hydroxide the complex salt is potassium tetrahydroxoaluminate(III) complex salt.
Al(OH) 3 (s) + KOH(aq) -> KAl(OH) 4(aq)
Ionic equation
Al(OH) 3(s) + OH-(aq) -> [Al(OH) 4]- (aq)
Summary of amphotellic oxides/Hydroxides
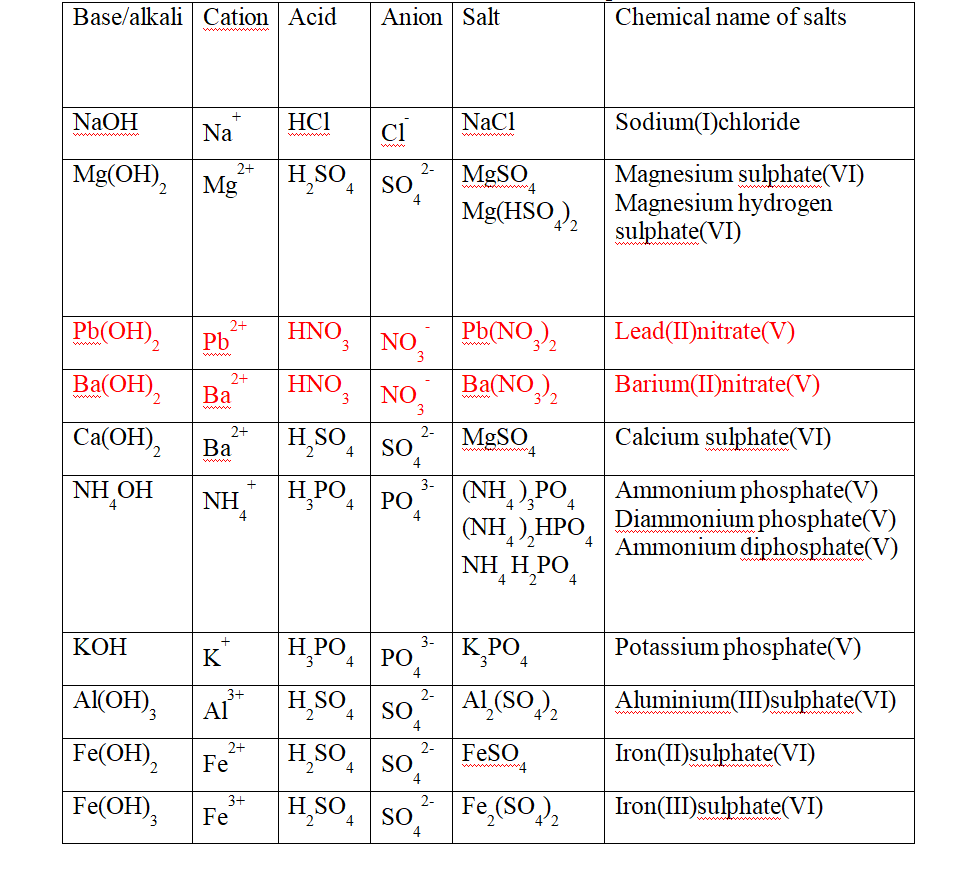
A salt is therefore formed when the hydrogen ions in an acid are replaced wHolly/fully or partially/partly ,directly or indirectly by a metal or ammonium radical.
(b) the number of ionizable/replaceable hydrogen in an acid is called basicity of an acid.
Some acids are therefore:
(i)monobasic acids generally denoted HX e.g.
HCl, HNO3,HCOOH,CHCOOH.
(ii)dibasic acids ; generally denoted H2X e.g.
H2SO4, H2SO3, H2CO3,HOocOOH.
(iii)tribasic acids ; generally denoted HX e.g.
HPO4.
(c) Some salts are normal salts wHile other are acid salts.
(i)A normal salt is formed when all the ionizable /replaceable hydrogen in an acid is replaced by a metal or metallic /ammonium radical.
(ii)An acid salt is formed when part/portion the ionizable /replaceable hydrogen in an acid is replaced by a metal or metallic /ammonium radical.
Table showing normal and acid salts derived from common acids
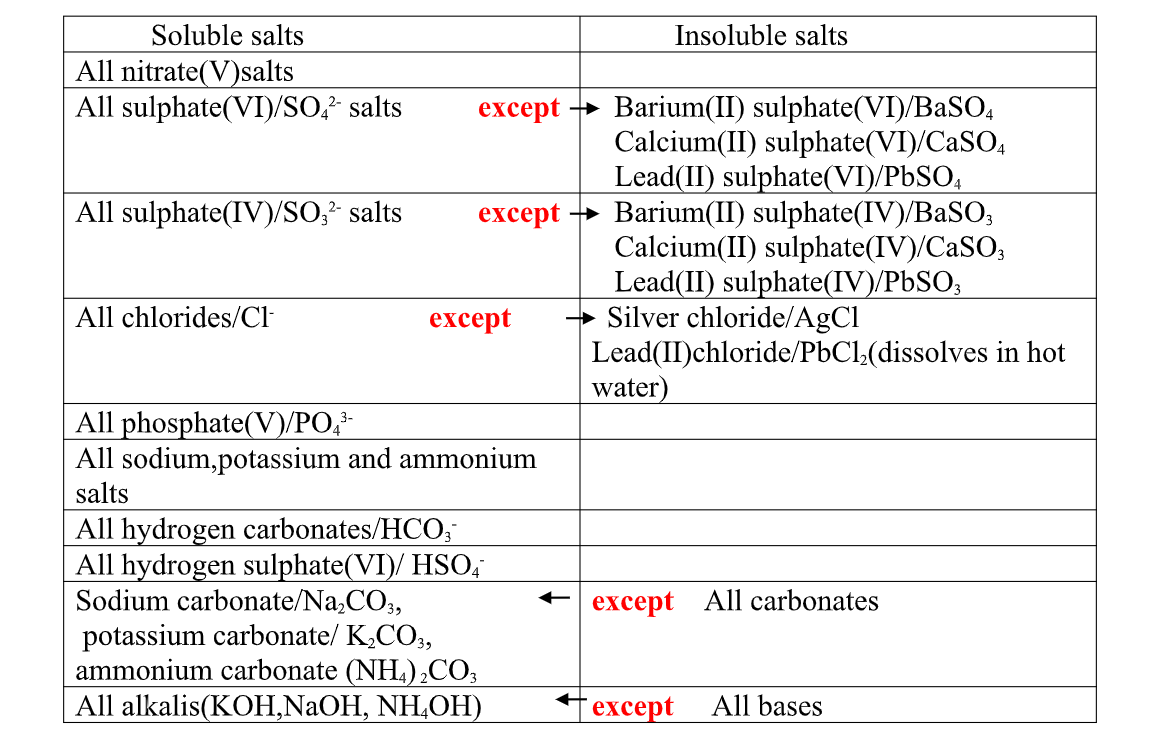
(i) Hygroscopic salts /compounds are those that absorb water from the atmosphere but do not form a solution.
Some salts which are Hygroscopic include anhydrous copper(II)Sulphate(VI), anhydrous cobalt(II)chloride, potassium nitrate(V) common table salt.
(ii)Deliquescent salts /compounds are those that absorb water from the atmosphere and form a solution.
Some salts which are deliquescent include: Sodium nitrate(V),Calcium chloride, Sodium Hydroxide, Iron(II)chloride, Magnesium chloride.
(iii)Efflorescent salts/compounds are those that lose their water of crystallization to the atmosphere.
Some salts which effloresces include: sodium carbonate decahydrate, Iron(II)Sulphate(VI)HeptaHydrate, sodium Sulphate (VI)decahydrate.
(e)Some salts contain water of crystallization.They are Hydrated.Others do not contain water of crystallization. they are anhydrous.
Table showing some Hydrated salts.
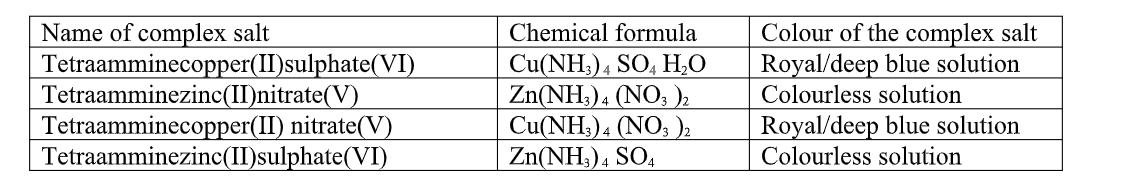
Table of some complex salts
(a) Soluble salts may be prepared by using any of the following methods:
(i)Direct displacement/reaction of a metal with an acid.
By reacting a metal higher in the reactivity series than hydrogen with a dilute acid,a salt is formed and hydrogen gas is evolved.
Excess of the metal must be used to ensure all the acid has reacted.
when effervescence/bubbling /fizzing has stopped ,excess metal is filtered.
the filtrate is heated to concentrate then allowed to crystallize.
WasHing with distilled water then drying between filter papers produces a sample crystal of the salt. i.e.
M(s) + H2X -> MX(aq) + H2(g)
Examples
(ii)Reaction of an insoluble base with an acid
By adding an insoluble base (oxide/Hydroxide )to a dilute acid until no more dissolves, in the acid,a salt and water are formed. Excess of the base is filtered off. the filtrate is heated to concentrate ,allowed to crystallize then washed with distilled water before drying between filter papers e.g.
(iii)reaction of insoluble /soluble carbonate /hydrogen carbonate with an acid.
By adding an excess of a soluble /insoluble carbonate or hydrogen carbonate to adilute acid, effervescence /fizzing/bubbling out of carbon(IV)oxide gas shows the reaction is taking place. when effervescence /fizzing/bubbling out of the gas is over, excess of the insoluble carbonate is filtered off. the filtrate is heated to concentrate ,allowed to crystallize then washed with distilled water before drying between filter paper papers e.g.


(iv)neutralization/reaction of soluble base/alkali with dilute acid
By adding an acid to a burette into a known volume of an alkali with 2-3 drops of an indicator, the colour of the indicator changes when the acid has completely reacted with an alkali at the end point.
The procedure is then repeated without the indicator .
The solution mixture is then heated to concentrate , allowed to crystallize ,washed with distilled water before drying with filter papers. e.g.
(iv)direct synthesis/combination.
when a metal burn in a gas jar containing a non metal , the two directly combine to form a salt. e.g.
Some salts once formed undergo sublimation and Hydrolysis. Care sHould be taken to avoid water/moisture into the reaction flask during their preparation.
SucH salts include aluminium(III)chloride(AlCl3) and iron (III)chloride(FeCl3)
1. heated aluminium foil reacts with Chlorine to form aluminium(III)chloride that sublimes away from the source of heating then deposited as solid again
Once formed aluminium(III)chloride Hydrolyses/reacts with water vapour / moisture present to form aluminium Hydroxide solution and highly acidic fumes of hydrogen chloride gas.
(b)Insoluble salts can be prepared by reacting two suitable soluble salts to form one soluble and one insoluble.
this is called double decomposition or precipitation.
The mixture is filtered and the residue is washed with distilled water then dried.
14. Salts may lose their water of crystallization , decompose ,melt or sublime on heating on a Bunsen burner flame.
the following shows the beHavior of some salts on heating gently /or strongly in a laboratory school burner:
(a)effect of Heat on chlorides
All chlorides havevery High melting and boiling points and therefore are not affected by laboratory heating except ammonium chloride.
Ammonium chloride sublimes on gentle heating. It dissociate into the constituent ammonia and hydrogen chloride gases on strong heating.
(i) Potassium nitrate(V)/KNO3 and sodium nitrate(V)/NaNO3 decompose on heating to form Potassium nitrate(III)/KNO2 and sodium nitrate(III)/NaNO2 and producing Oxygen gas in eachcase.
(ii)Heavy metal nitrates(V) salts decompose on heating to form the oxide and a mixture of brown acidic nitrogen(IV)oxide and oxygen gases. e.g.
(iii)Silver(I)nitrate(V) and mercury(II) nitrate(V) are lowest in the reactivity series. they decompose on heating to form the metal(silver and mercury)and the Nitrogen(IV)oxide and oxygen gas. i.e.
(iv)Ammonium nitrate(V) and Ammonium nitrate(III) decompose on heating to Nitrogen(I)oxide(relights/rekindles glowing splint) and nitrogen gas respectively.Water is also formed.i.e.
(c) effect of Heat on nitrate(V)
Only Iron(II)Sulphate(VI), Iron(III)Sulphate(VI) and copper(II)Sulphate(VI) decompose on heating. they form the oxide, and produce highly acidic fumes of acidic sulpHur(IV)oxide gas.
(d) effect of Heat on carbonates(IV) and hydrogen carbonate(IV).
(i)Sodium carbonate(IV)and potassium carbonate(IV)do not decompose on heating.
(ii)Heavy metal nitrate(IV)salts decompose on heating to form the oxide and produce carbon(IV)oxide gas.
Carbon (IV)oxide gas forms a white precipitate when bubbled in lime water. the white precipitate dissolves if the gas is in excess. e.g.
(iii)Sodium hydrogen carbonate(IV) and Potassium hydrogen carbonate(IV)decompose on heating to give the corresponding carbonate (IV) and form water and carbon(IV)oxide gas. i.e.
(iii) Calcium hydrogen carbonate (IV) and Magnesium hydrogen carbonate(IV) decompose on heating to give the corresponding carbonate (IV) and form water and carbon(IV)oxide gas. i. e.
the cation and anion in a salt is determined/known usually by precipitation of the salt using a precipitating reagent.
the colour of the precipitate is a basis of qualitative analysis of a compound.
16.Qualitative analysis is the process of identifying an unknown compound /salt by identifying the unique qualities of the salt/compound.
It involves some of the following processes.
(a)Reaction of cation with sodium/potassium Hydroxide solution.
both sodium/potassium Hydroxide solutions are precipitating reagents.
the alkalis produce unique colour of a precipitate/suspension when a few/three drops is added and then excess alkali is added to unknown salt/compound solution.
NB: Potassium Hydroxide is not commonly used because it is more expensive than sodium Hydroxide.
the table below shows the observations, inferences / deductions and explanations from the following test tube experiments:
Procedure
Put about 2cm3 of MgCl2, CaCl2, AlCl3, NaCl, KCl, FeSO 4, Fe2(SO 4) 3, CuSO 4, ZnSO 4NH4NO3, Pb(NO3) 2, Ba(NO3) 2 eachinto separate test tubes. Add three drops of 2M sodium Hydroxide solution then excess (2/3 the length of a standard test tube).
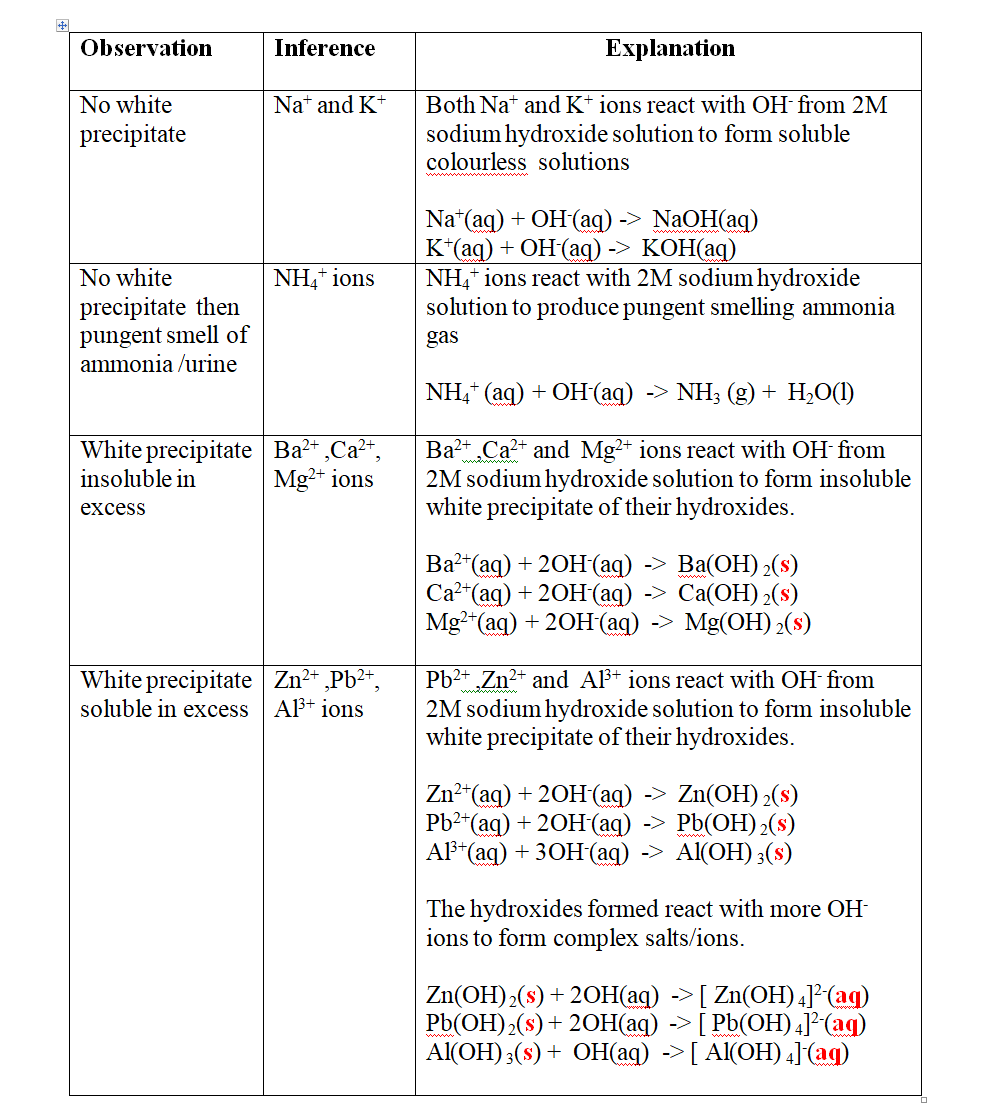
cu2+(aq) + 2OH-(aq) -> Cu(OH) 2(s)
Green precipitate insoluble in excess
On adding 3cm3 of hydrogen peroxide, brown/yellow solution formed Fe2+ Fe2+ oxidized to Fe3+ Fe2+ ions react with OH- from 2M sodium Hydroxide solution to form insoluble green precipitate of Iron(II) Hydroxide.
Fe2+(aq) + 2OH-(aq) -> Fe(OH) 2(s) hydrogen peroxide is an oxidizing agent that oxidizes green Fe2+ oxidized to brown Fe3+ Fe(OH) 2(s) + 2H+ -> Fe(OH) 3(aq)
Brown precipitate insoluble in excess Fe3+ Fe3+ ions react with OH- from 2M sodium Hydroxide solution to form insoluble brown precipitate of Iron(II) Hydroxide. Fe3+(aq) + 3OH-(aq) -> Fe(OH) 3(s)
(b)Reaction of cation with aqueous ammonia
Aqueous ammonia precipitating reagent that can be used to identify the cations present in a salt.
Like NaOH/KOH the OH- ion in NH4OH react with the cation to form a cHaracteristic Hydroxide .
Below are the observations ,inferences and explanations of the reactions of aqueous ammonia with salts from the following test tube reactions.
Procedure
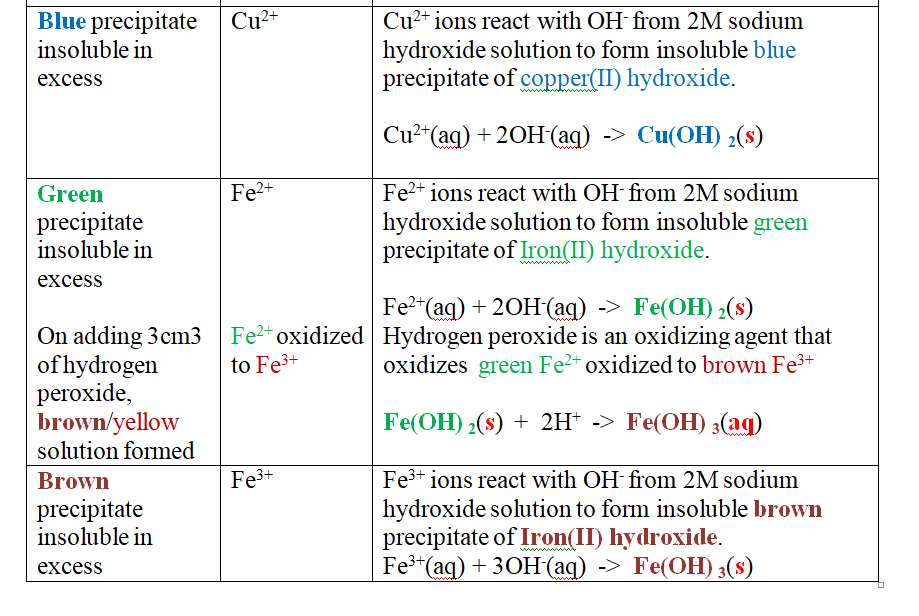
Put about 2cm3 of MgCl2, CaCl2, AlCl3, NaCl, KCl, FeSO 4, Fe2(SO 4) 3, CuSO 4, ZnSO 4NH4NO3, Pb(NO3) 2, Ba(NO3) 2 eachinto separate test tubes. Add three drops of 2M aqueous ammonia then excess (2/3 the length of a standard test tube).
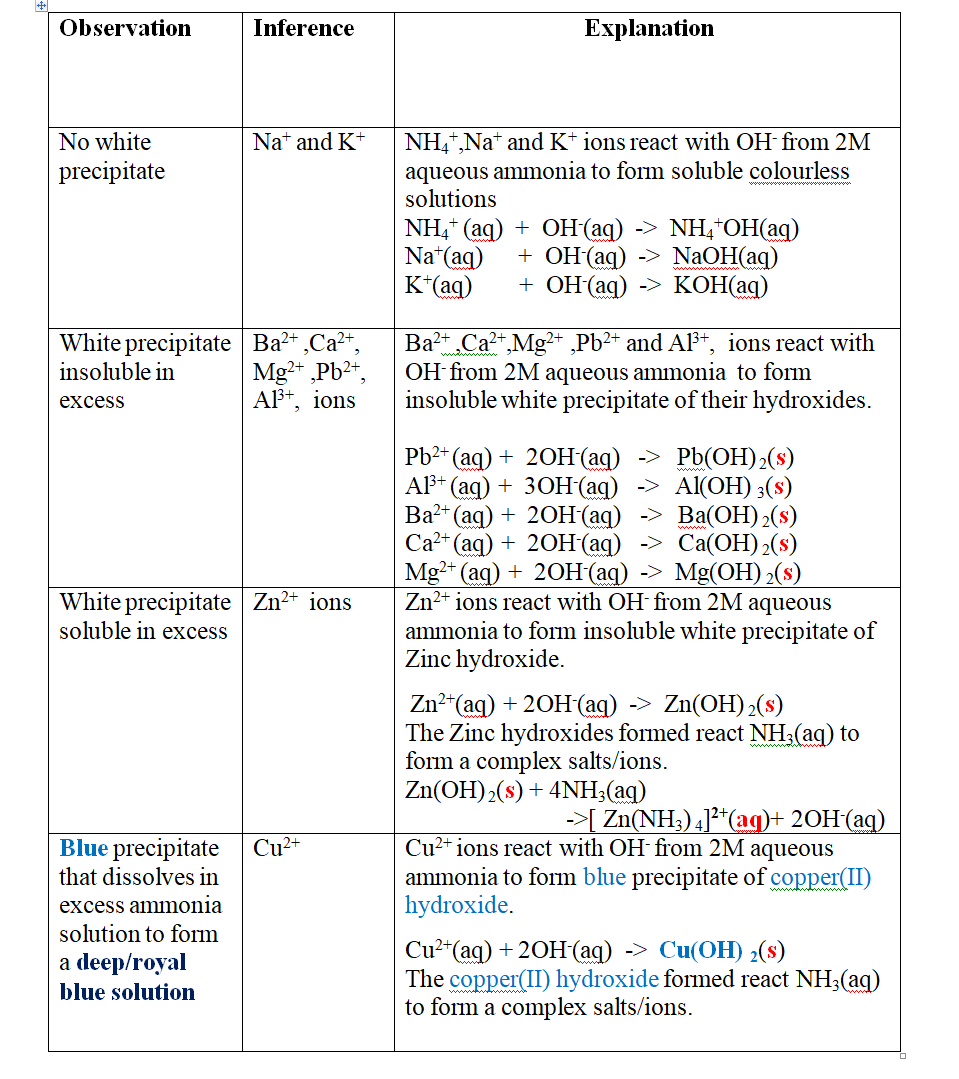
Green precipitate insoluble in excess.
On adding 3cm3 of hydrogen peroxide, brown/yellow solution formed Fe2+ Fe2+ oxidized to Fe3+ Fe2+ ions react with OH- from 2M aqueous ammonia to form insoluble green precipitate of Iron(II) Hydroxide.
Note
(i) Only zn2+ ions/salts form a white precipitate that dissolve in excess of both 2M sodium Hydroxide and 2M aqueous ammonia.
(ii) pb2+ and Al3+ ions/salts form a white precipitate that dissolve in excess of 2M sodium Hydroxide but not in 2M aqueous ammonia.
(iii) cu2+ ions/salts form a blue precipitate that dissolve to form a deep/royal blue solution in excess of 2M aqueous ammonia but only blue insoluble precipitate in 2M sodium Hydroxide
(c)Reaction of cation with chloride (Cl-)ions
All chlorides are soluble in water except Silver chloride and Lead (II)chloride (that dissolve in hot water).when a soluble chloride like NaCl, KCl, NH4Cl is added to about 2cm3 of a salt containing Ag+ or pb2+ions a white precipitate of AgCl or PbCl2 is formed.
the following test tube reactions illustrate the above.
Experiment Put about 2cm3 of silver nitrate(V) andLead(II)nitrate(V)solution into separate test tubes. Add five drops of NaCl /KCl / NH4Cl/HCl. Heat to boil.
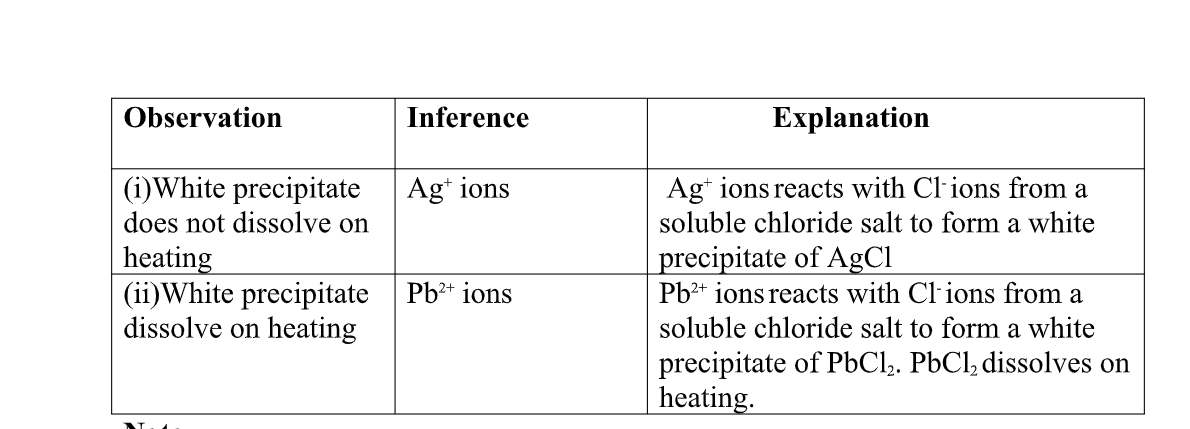
both pb2+ and Al3+ ions forms an insoluble white precipitate in excess aqueous ammonia. A white precipitate on adding Cl-ions/salts shows pb2+.
No white precipitate on adding Cl-ions/salts shows Al3+. Adding a chloride/ Cl-ions/salts can thus be used to separate the identity of Al3+ and pb2+.
(d)Reaction of cation with Sulphate(VI)/SO 42- and Sulphate(IV)/SO32- ions
All Sulphate(VI) and Sulphate(IV)/SO32- ions/salts are soluble/dissolve in water except Calcium Sulphate(VI)/CaSO 4, Calcium Sulphate(IV)/CaSO3, Barium Sulphate(VI)/BaSO 4, Barium Sulphate(IV)/BaSO3, Lead(II) Sulphate(VI)/PbSO 4 and Lead(II) Sulphate(IV)/PbSO3.
when a soluble Sulphate(VI)/SO 42- salt like Na2SO 4, H2SO 4, (NH4)2SO 4 or Na2SO3 is added to a salt containing Ca2+, pb2+, Ba2+ ions, a white precipitate is formed.
the following test tube experiments illustrate the above.
Procedure
Place about 2cm3 of Ca(NO3) 2, Ba(NO3) 2, BaCl2 and Pb(NO3) 2, in separate boiling tubes. Add six drops of Sulphuric(VI)acid /sodium Sulphate(VI)/ammonium Sulphate(VI)solution. Repeat with six drops of sodium Sulphate(IV).
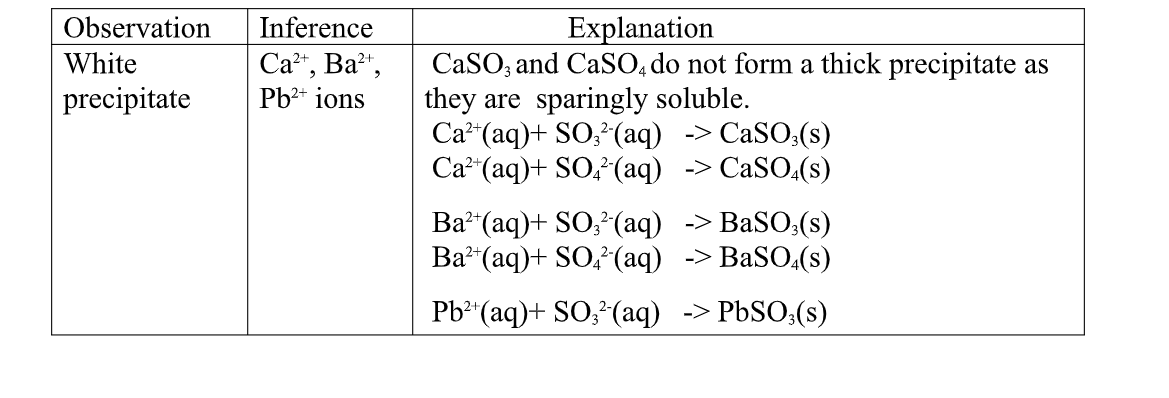
All carbonate salts are insoluble except sodium/potassium carbonate(IV) and ammonium carbonate(IV).
they dissociate /ionize to release CO32- ions. CO32- ions produce a white precipitate when the soluble carbonate salts is added to any metallic cation.
Procedure
Place about 2cm3 of Ca(NO3) 2, Ba(NO3) 2, MgCl2 ,Pb(NO3) 2 andZnSO 4 in separate boiling tubes.
Add six drops of Potassium /sodium carbonate(IV)/ ammonium carbonate (IV)solution.
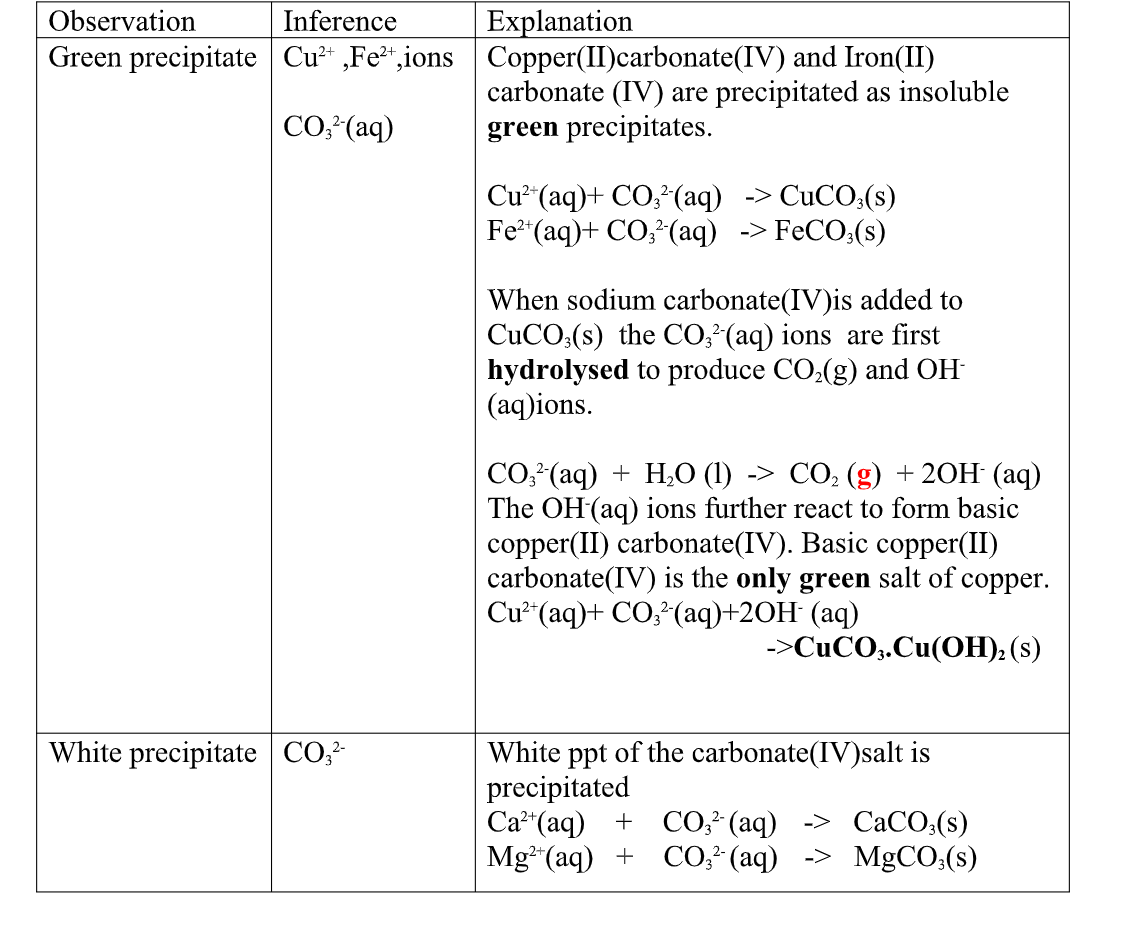
(ii)Copper(II)Carbonate(IV) exist only as the basic CuCO3.Cu(OH) 2
(iii)both BaCO3 and BaSO3 are insoluble white precipitate.
If Hydrochloric acid is added to the white precipitate;
I. BaCO3 produces CO2 gas. when bubbled/directed into lime water solution,a white precipitate is formed. II. I. BaSO3 produces SO2 gas.
when bubbled/directed into orange acidified potassium dichromate(VI) solution, it turns to green/decolorizes acidified potassium manganate(VII).
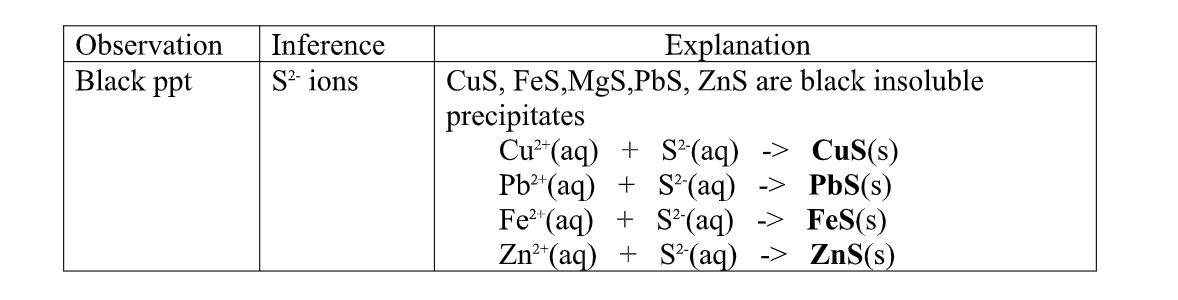
(f) Reaction of cation with sulphide / S2- ions
All sulphides are insoluble black solids/precipitates except sodium sulphide/ Na2S/ potassium sulphide/K2S.when a few/3drops of the soluble sulphide is added to a metal cation/salt, a black precipitate is formed.
Procedure
Place about 2cm3 of Cu(NO3) 2, FeSO 4, MgCl2,Pb(NO3) 2 and ZnSO 4 in separate boiling tubes.
Add six drops of Potassium /sodium sulphide solution.
You are provided with solid Y(aluminium (III)Sulphate(VI)Hexahydrate).
Carry out the following tests and record your observations and inferences in the space provided.
1(a) Appearance
Observations inference (1mark) white crystalline solid Coloured ions cu2+ , Fe2+ ,Fe3+ absent
(b)Place about a half spatula full of the solid into a clean dry boiling tube. Heat gently then strongly.
Observations inference
(1mark) Colourless droplets formed on the cooler Hydrated compound/compound part of the test tube containing water of crystallization Solid remains a white residue
(c)Place all the remaining portion of the solid in a test tube .Add about 10cm3 of distilled water. Shake thoroughly. Divide the mixture into five portions. Observation Inference (1mark) Solid dissolves to form Polar soluble compound
a colourless solution cu2+ , Fe2+ ,Fe3+ absent
(i)To the first portion, add three drops of sodium Hydroxide then add excess of the alkali.
Observation Inference (1mark) white ppt, soluble in excess zn2+ , pb2+ , Al3+
(ii)To the second portion, add three drops of aqueous ammonia then add excess of the alkali.
Observation Inference (1mark) white ppt, insoluble in excess pb2+ , Al3+
(iii)To the tHird portion, add three drops of sodium Sulphate(VI)solution.
Observation Inference (1mark) No white ppt
Al3+
(iv)I.To the fourthportion, add three drops of Lead(II)nitrate(IV)solution. Preserve
Observation Inference (1mark) white ppt CO32-, SO 42-, SO32-, Cl-,
II.To the portion in (iv) I above , add five drops of dilute Hydrochloric acid.
Observation Inference
(1mark) white ppt persist/remains SO 42-, Cl-,
III.To the portion in (iv) II above, Heat to boil.
Observation Inference (1mark) white ppt persist/remains SO 42-,
Note that:
(i) From test above, it can be deduced that solid Y is Hydrated aluminium(III)Sulphate(VI) solid
(ii) Any ion inferred from an observation below must be derived from previous correct observation and inferences above. e.g. Al3+ in c
(iii) must be correctly inferred in either/or in c(ii) or c(i)above
SO 42- in c
(iv)III must be correctly inferred in either/or in c(iv)II or c(iv)I above
(iii) Contradiction in observations and inferences should be avoided.e.g.
“white ppt soluble in excess” to infer presence of Al3+ ,Ba2+ ,pb2+
(iv) Symbols of elements/ions should be correctly capitalized. e.g.
“SO 4-2” is wrong, “SO 42-” is wrong, “cu2+” is wrong.
Sample solutions of salt were labeled as I,II, III and IV. the actual solutions, not in that order are lead nitrate, zinc Sulphate potassium chloride and calcium chloride.
a) when aqueous sodium carbonate was added to eachsample separately, a white precipitate was formed in I, III and IV only. Identify solution II.
b) when excess sodium Hydroxide was added to eachsample separately, a white precipitate was formed in solutions III and I only.
Identify solution I
17.when solids/salts /solutes are added to a solvent ,some dissolve to form a solution.
Solute + Solvent -> Solvent
If a solution has a lot of solute dissolved in a solvent ,it is said to be concentrated.
If a solution has little solute dissolved in a solvent ,it is said to be dilute.
there is a limit to How mucH solute can dissolve in a given /specified amount of solvent/water at a given /specified temperature.
the maximum mass of salt/solid/solute that dissolve in 100g of solvent/water at a specified temperature is called solubility of a salt.
when no more solute can dissolve in a given amount of solvent at a specified temperature, a saturated solution is formed.
For some salts, on heating, more of the salt/solid/solute dissolve in the saturated solution to form a super saturated solution.
The solubility of a salt is thus calculated from the formula
Solubility = Mass of solute/salt/solid x 100 Mass/volume of water/solvent
Practice examples
(a) Calculate the solubility of potassium nitrate(V) if 5.0 g of the salt is dissolved in 50.0cm3 of water.
Solubility = Mass of solute/salt/solid x 100 =>( 5.0 x 100 ) = 10.0 g /100g H 2O Mass/volume of water/solvent 50.0
(b) Calculate the solubility of potassium cHlorate(V) if 50.0 g of the salt is dissolved in 250.0cm3 of water.
Solubility = Mass of solute/salt/solid x 100 =>( 50.0 x 100 ) = 20.0 g /100g H 2O Mass/volume of water/solvent 250.0
(c) If the solubility of potassium cHlorate(V) is 5g/100g H 2O at 80oC,How mucH can dissolve in 5cm3 of water at 80oC .
Mass of solute/salt/solid = Solubility x Mass/volume of water/solvent 100 => 5 x 5 = 0.25g of KClO3 dissolve 100
(d) If the solubility of potassium cHlorate(V) is 72g/100g H 2O at 20oC,How mucH can saturate 25g of water at 20oC .
Mass of solute/salt/solid = Solubility x Mass/volume of water/solvent 100 => 72 x 25 = 18.0g of KClO3 dissolve/saturate 100
(e) 22g of potassium nitrate(V) was dissolved in 40.0g of water at 10oC. Calculate the solubility of potassium nitrate(V) at 10oC.
Solubility = Mass of solute/salt/solid x 100 =>( 22 x 100 ) = 55.0 g /100g H 2O Mass/volume of water/solvent 40.0.
(f)what volume of water should be added to 22.0g of water at 10oC if the solubility of KNO3 at 10oC is 5.0g/100g H 2O?
Solubility is mass/100g H 2O => 22.0g + x = 100cm3/100g H 2O X= 100 – 22 = 78 cm3 of H 2O 18. A graph of solubility against temperature is called solubility curve.
It shows the influence of temperature on solubility of different substances/solids/salts.
Some substances dissolve more with increase in temperature while for others dissolve less with increase in temperature
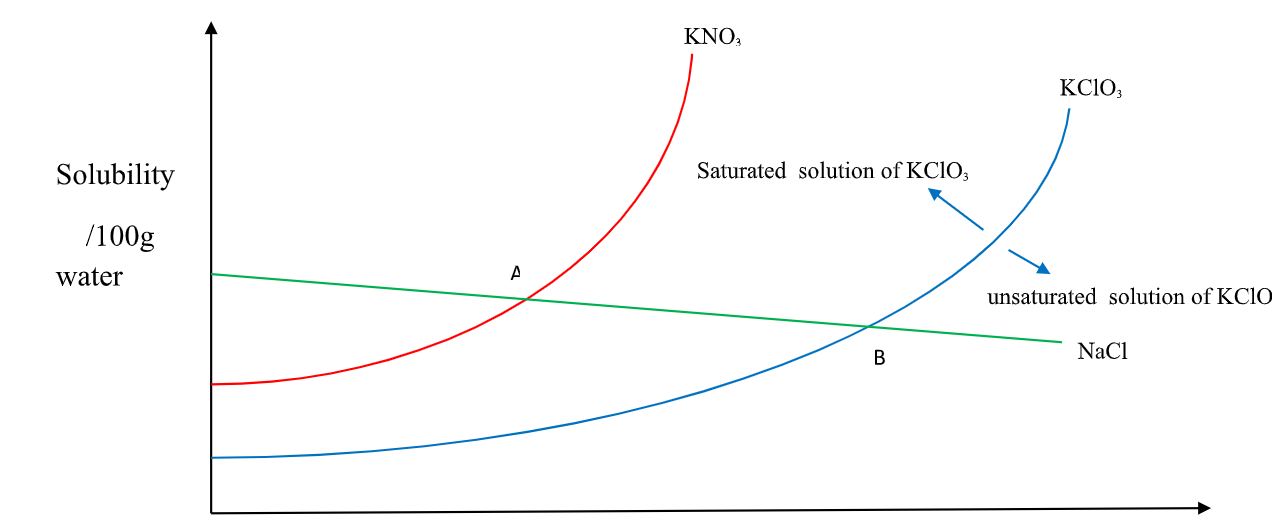
(i) Solubility of KNO3 and KClO3 increase with increase in temperature.
(ii) Solubility of KNO3 is always higher than that of KClO3 at any specified temperature.
(iii) Solubility of NaCl decrease with increase in temperature.
(iv)NaCl has the Highest solubility at low temperature while KClO3 has the lowest solubility at low temperature.
(v) At point A both NaCl and KNO3 are equally soluble.
(vi) At point B both NaCl and KClO3 are equally soluble.
(vii) An area above the solubility curve of the salt shows a saturated /supersaturated solution.
(viii) An area below the solubility curve of the salt shows an unsaturated solution.
19.(a) For salts whose solubility increases with increase in temperature, crystals form when the salt solution at higher temperatures is cooled to a lower temperature.
(b) For salts whose solubility decreases with increase in temperature, crystals form when the salt solution at lower temperatures is heated to a higher temperature.
the examples below shows determination of the mass of crystals deposited with changes in temperature.
1.The solubility of KClO3 at 100oc is 60g/100g water .what mass of KClO3 will be deposited at:
(i)75 oc if the solubility is now 39g/100g water.
At 100oc = 60.0g
Less at 75oc = - 39.0g
Mass of crystallized out 21.0g
(i)35 oc if the solubility is now 28 g/100g water.
At 100oc = 60.0g
Less at 35oC = - 28.0.0g
Mass of crystallized out 32.0g
2. KNO3 has a solubility of 42 g/100g water at 20oC.The salt was heated and added 38g more of the solute which dissolved at100oc.
Calculate the solubility of KNO3 at 100oc.
Solubility of KNO3 at 100oc = solubility at 20oC + mass of KNO3 added
=> 42g + 38g = 80g KNO3 /100g H 2O
3. A salt solution has a mass of 65g containing 5g of solute. the solubility of thissalt is 25g per 100g water at 20oC. 60g of the salt are added to the solution at 20oC.Calculate the mass of the solute that remain undissolved.
Mass of solvent at 20oC = mass of solution – mass of solute
=> 65 - 5 = 60g
Solubility before adding salt = mass of solute x 100
Volume of solvent
=> 5 x 100 = 8.3333g/100g water 60
Mass of solute to equalize with solubility = 25 – 8.3333g = 16.6667g
Mass of solute undissolved = 60.0 - 16.6667g = 43.3333 g
4. Study the table below
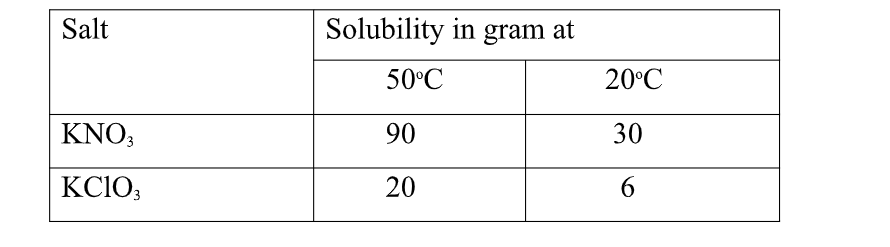
(90 – 30) = 60.0 g of KNO3 crystals precipitate
(20 – 6) = 14.0 g of KClO3 crystals precipitate
(ii)State the assumption made in (i) above.
Solubility of one salt has no effect on the solubility of the other.
5. 10.0 g of Hydrated potassium carbonate (IV) K2CO3.xH 2O on heating leave 7.93 of the Hydrate.
(a)Calculate the mass of anhydrous salt obtained.
Hydrated on heating leave anhydrous = 7.93 g
(b)Calculate the mass of water of crystallization in the Hydrated salt
Mass of water of crystallization = Hydrated – anhydrous
=> 10.0 - 7.93 = 2.07 g
(c)How many moles of anhydrous salt are there in 10of Hydrate? (K= 39.0,C=12.0.O= 16.0)
Molar mass K2CO3= 138
Moles K2CO3 = mass of K2CO3 => 7.93 = 0.0515 moles
Molar mass K2CO3 138 (d)How many moles of water are present in the Hydrate for every one mole of K2CO3 ? (H=1.0.O= 16.0)
Molar mass H 2O = 18
Moles H 2O = mass of H 2O => 2.07 = 0.115 moles Molar mass H 2O 18
Mole ratio H 2O : K2CO3 = 0.115 moles 2 = 2 0.0515 moles 1 (e)what is the formula of the Hydrated salt?
K2CO3 .2 H 2O
6. the table below shows the solubility of Potassium nitrate(V) at different temperatures.
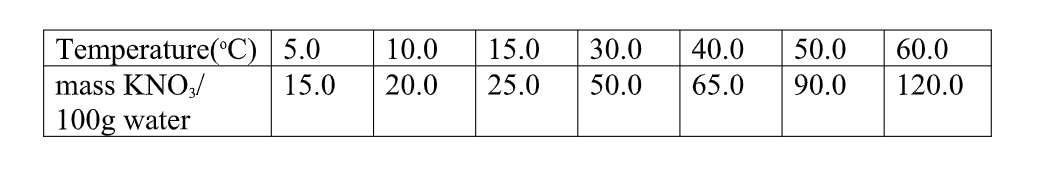
(i)the mass of KNO3 dissolved at:
I. 20oC From a correctly plotted graph = 32g II. 35oC
From a correctly plotted graph = 57g
III. 55oC
From a correctly plotted graph = 104g
(ii)the temperature at which the following mass of KNO3 dissolved:
I. 22g
From a correctly plotted graph =13.0oC
II. 30g
From a correctly plotted graph =17.5oC
III.100g
From a correctly plotted graph =54.5oC
(c)Explain the shape of your graph.
Solubility of KNO3 increase with increase in temperature/More KNO3 dissolve as temperature rises.
(d)show on the graph the supersaturated and unsaturated solutions.
Above the solubility curve write; “supersaturated”
Below the solubility curve write; “unsaturated”
(e)From your graph, calculate the amount of crystals obtained when a saturated solution of KNO3 containing 180g of the salt is cooled from 80oC to:
I. 20oC
Solubility before heating = 180 g
Less Solubility after heating(from the graph) = 32 g
Mass of KNO3crystals = 148 g
II. 35oC
Solubility before heating = 180 g
Less Solubility after heating(from the graph) = 58 g
Mass of KNO3crystals = 122 g
III. 55oC
Solubility before heating = 180 g
Less Solubility after heating(from the graph) = 102 g
Mass of KNO3crystals = 78 g
7. the table below shows the solubility of salts A and B at various temperatures.
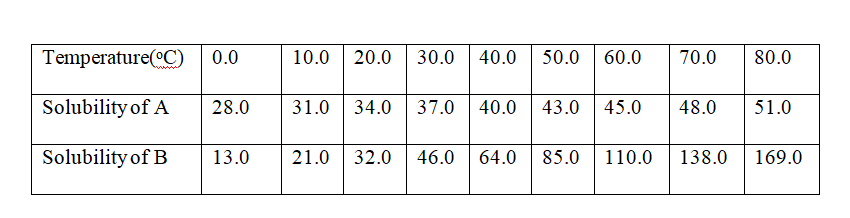
the point of intersection of the two curves = 24oc
(c)what Happens when a mixture of 100g of salt B with 100g if water is heated to 80oC
From the graph, the solubility of B at 80oC is 169g /100g water. All the 100g crystals of B dissolve.
(d)what Happens when the mixture in (c) above is then cooled from 50oC to 20oC.
method I.
Total mass before cooling at 50oC = 100.0 g
(From graph) Solubility/mass after cooling at 20oC = 32.0 g
Mass of crystals deposited 68.0 g
method II.
Mass of soluble salt crystals at 50oC added = 100 g
(From graph)Solubility/mass before cooling at 50oC = 85.0 g
Mass of crystals that cannot dissolve at 50oC 15.0 g
(From graph) Solubility/mass before cooling at 50oC = 85.0 g
(From graph) Solubility/mass after cooling at 20oC = 32.0 g
Mass of crystals deposited after cooling 53.0 g
Total mass of crystals deposited = 15.0 + 53.0 = 68.0 g
(e)A mixture of 40g of A and 60g of B is added to 10g of water and heated to 70oc.
The solution is then allowed to cool to 10oC.Describe clearly what Happens.
I.For salt A
Solubility of A before heating = mass of A x 100
Volume of water added
=> 40 x 100 = 400g/100g Water
10
(theoretical)Solubility of A before heating = 400 g
Less (From graph ) Solubility of A after heating at 70oc = 48g
Mass of crystals that can not dissolve at70oc
= 352 g
(From graph ) Solubility of A after heating at 70oc = 48g
Less (From graph ) Solubility of A after cooling to 10oC = 31g
Mass of crystals that crystallize out on cooling to10oC = 17 g
Mass of crystals that can not dissolve at70oc
= 352 g
Add Mass of crystals that crystallize out on cooling to10oC = 17 g
Total mass of A that does not dissolve/crystallize/precipitate = 369 g
I.For salt B
Solubility of B before heating = mass of B x 100
Volume of water added
=> 60 x 100 = 600g/100g Water
10
(theoretical)Solubility of B before heating = 600 g
Less (From graph ) Solubility of B after heating at 70oc = 138g
Mass of crystals that cannot dissolve at70oc = 462 g
(From graph ) Solubility of B after heating at 70oc = 138g
Less (From graph ) Solubility of B after cooling to 10oC = 21g
Mass of crystals that crystallize out on cooling to10oC = 117 g
Mass of crystals that cannot dissolve at70oc = 462 g
Add Mass of crystals that crystallize out on cooling to10oC = 117 g
Total mass of A that does not dissolve/crystallize/precipitate = 579 g
(f)State the assumption made in (e)above
Solubility of one salt has no effect on the solubility of the other
8. when 5.0 g of potassium cHlorate (V) was put in 10cm3 of water and heated, the solid dissolves.
when the solution was cooled , the temperature at which crystals reappear was noted.
Another 10cm3 of water was added and the mixture heated to dissolve then cooled for the crystals to reappear .
The table below shows the the results obtained
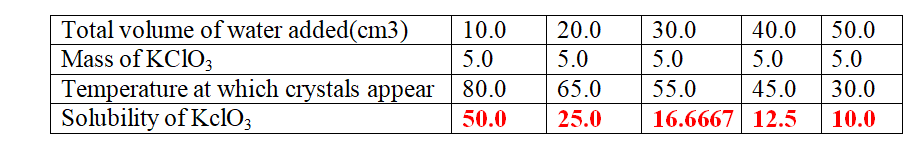
(b)Plot a graph of mass of KClO3 per 100g water against temperature at which crystals form.
(c)From the graph, show and determine ;
(i)the solubility of KClO3 at
I. 50oC
From a well plotted graph = 14.5 g KClO3/100g water
II. 35oC
From a well plotted graph = 9.0 g KclO3/100g water
(ii)the temperature at which the solubility is:
I.10g/100g water
From a well plotted graph = 38.0 oc
II.45g/100g water
From a well plotted graph = 77.5 oc
(d)Explain the shape of the graph.
Solubility of KClO3 increase with increase in temperature/more KclO3dissolve as temperature rises.
(e)what Happens when 100g per 100g water is cooled to 35.0 oc
Solubility before heating = 100.0
(From the graph) Solubility after cooling = 9.0
Mass of salt precipitated/crystallization = 91.0 g
9. 25.0cm3 of water dissolved various masses of ammonium chloride crystals at different temperatures as shown in the table below.
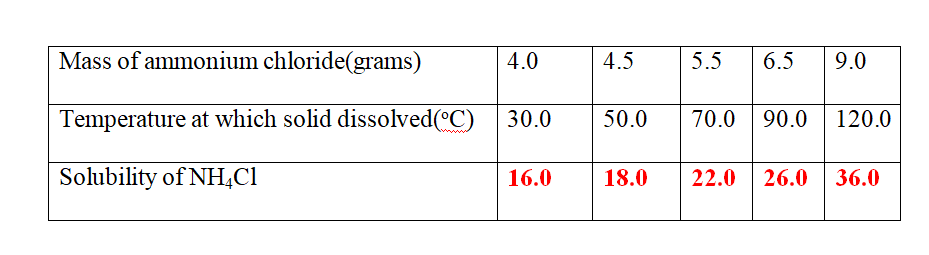
(b)Plot a solubility curve
(c)what Happens when a saturated solution of ammonium chloride is cooled from 80oC to 40oc.
(From the graph )Solubility at 80oC = 24.0 g
Less (From the graph )Solubility at 40oc = 16.8 g
Mass of crystallized/precipitated = 7.2 g
20. Solubility and solubility curves are therefore used
(i) to know the effect of temperature on the solubility of a salt
(ii)to fractional crystallize two soluble salts by applying their differences in solubility at different temperatures.
(iii)determine the mass of crystal that is obtained from crystallization.
21.Natural fractional crystallization takes place in Kenya/East Africa at:
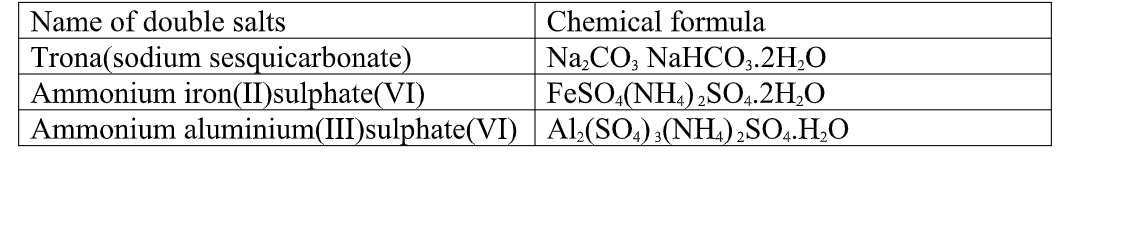
(i) Lake Magadi during extraction of soda asH(Sodium carbonate) from Trona(sodium sesquicarbonate)
(ii) Ngomeni near Malindi at the Indian ocean Coastline during the extraction of common salt(sodium chloride).
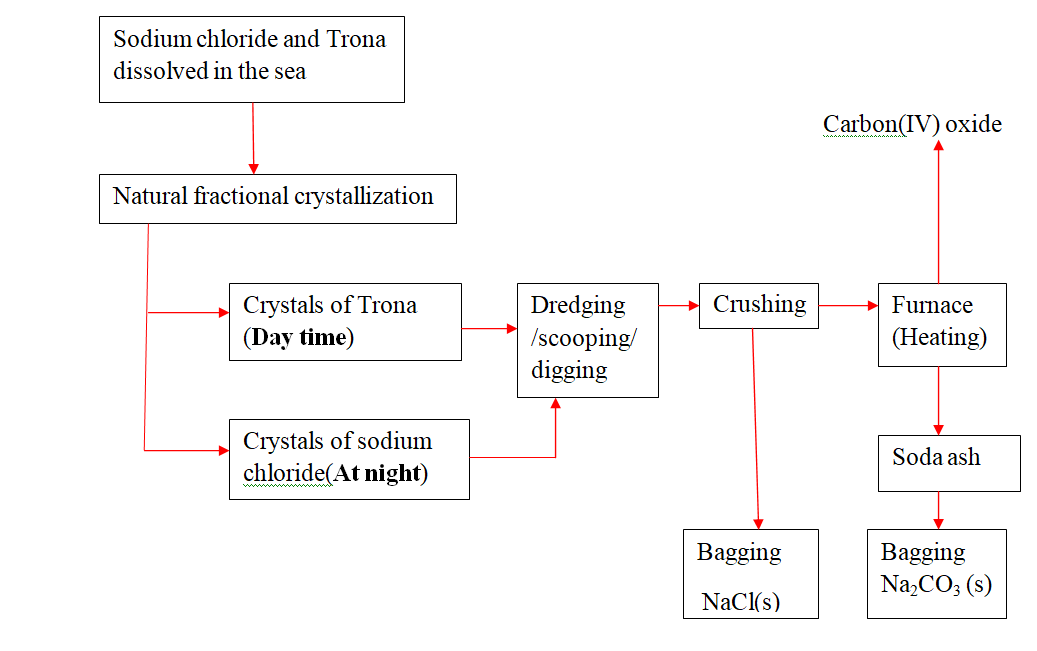
22.Extraction of soda asH from Lake Magadi in Kenya
Rain water drains underground in the great rift valley and percolate underground where it is heated geothermically.
the hot water dissolves underground soluble sodium compounds and comes out on the surface as alkaline springs which are found around the edges of Lake Magadi in Kenya.
Temperatures around the lake are very High (30-40oc) during the day.
the solubility of trona decrease with increase in temperature therefore solid crystals of trona grows on top of the lake (upto or more than 30metres tHick)
A bucket dredger mines the trona which is then crusHed ,mixed with lake liquor and pumped to wasHery plant where it is further refined to a green granular product called CRS.
the CRS is then heated to chemically decompose trona to soda asH(Sodium carbonate)
chemical equation
2Na2CO3.NaHCO3.2H 2O(s) -> 3Na2CO3 (s) + CO2(g) + 5H 2O(l) Soda asH(Sodium carbonate) is then stored .It is called Magadi Soda. Magadi Soda is used :
(i) make glass
(ii) for making soapless detergents
(iii) softening Hard water.
(iv) Common salt is colledcted at nigHt because its solubility decreases with decrease in temperature. It is used as salt lick/feed for animals.
Summary flow diagram showing the extraction of Soda asH from Trona
They contain a variety of dissolved salts (about 77% being sodium chloride).
During High tide ,water is collected into shallow pods and allowed to crystallize as evaporation takes place.The pods are constructed in series to increase the rate of evaporation.
At the final pod ,the crystals are scapped together,piled in a Heap and washed with brine (concentrated sodium chloride).
It contains MgCl2 and CaCl2 . MgCl2 and CaCl2are Hygroscopic. they absorb water from the atmosphere and form a solution.
this makes table salt damp/wet on exposure to the atmosphere.
24.Some water form lather easily with soap wHile others do not.
Water which form lather easily with soap is said to be “soft”
Water which do not form lather easily with soap is said to be “Hard”
Hardness of water is caused by the presence of Ca2+ and Mg2+ ions.
Ca2+ and Mg2+ ions react with soap to form an insoluble grey /white suspension/precipitate called Scum/ curd.
Ca2+ and Mg2+ ions in water come from the water sources passing through rocks containing soluble salts of Ca2+ and Mg2+ e.g. Limestone or gypsum
there are two types of water Hardness:
(a)temporary Hardness of water
(b)permanent Hardness of water
(a)temporary Hardness of water
Temporary Hardness of water is caused by the presence of dissolved calcium hydrogen carbonate/Ca(HCO3)2 and magnesium hydrogen carbonate/Mg(HCO3)2 when rain water dissolve carbon(IV) oxide from the air it forms waek carbonic(IV) acid i.e. CO2(g) + H 2O(l) -> H2CO3(aq) when carbonic(IV) acid passes throughlimestone/dolomite rocks it reacts to form soluble salts i.e. In limestone areas; H2CO3(aq) + CaCO3(s) -> Ca(HCO3)2 (aq) In dolomite areas; H2CO3(aq) + MgCO3(s) -> Mg(HCO3)2 (aq)
(b)permanent Hardness of water
Permanent Hardness of water is caused by the presence of dissolved calcium Sulphate(VI)/CaSO 4 and magnesium Sulphate(VI)/Mg SO 4 Permanent Hardness of water is caused by water dissolving CaSO 4 and MgSO 4 from ground rocks.
Hardness of water can be removed by the following methods:
(a)Removing temporary Hardness of water
(i)Boiling/heating.
Boiling decomposes insoluble calcium hydrogen carbonate/Ca(HCO3)2 and magnesium hydrogen carbonate/Mg(HCO3)2 to insoluble CaCO3 and MgCO3 that precipitate away. i.e
chemical equation
Ca(HCO3)2(aq) -> CaCO3 (s) + CO2(g) + H 2O(l)
Mg(HCO3)2(aq) -> MgCO3 (s) + CO2(g) + H 2O(l)
(ii)Adding sodium carbonate (IV) /WasHing soda.
Since boiling is expensive on a large scale ,a calculated amount of sodium carbonate decahydrate /Na2CO3.10H 2O precipitates insoluble Ca2+(aq) and Mg2+(aq) ions as carbonates to remove both temporary and permanent Hardness of water .
Thisa double decomposition reaction where two soluble salts form an insoluble and soluble salt. i.e.
(i)with temporary Hard water
chemical equation
Na2CO3 (aq) + Ca(HCO3) 2 (aq) -> NaHCO3(aq) + CaCO3 (s)
Na2CO3 (aq) + Mg(HCO3) 2 (aq) -> NaHCO3(aq) + MgCO3 (s)
Ionic equation
CO32- (aq) + Ca2+ (aq) -> CaCO3 (s) CO32- (aq) + Mg2+ (aq) -> MgCO3 (s)
(ii)with permanent Hard water
chemical equation
Na2CO3 (aq) + MgSO 4 (aq) -> Na2SO 4 (aq) + MgCO3 (s) Na2CO3 (aq) + CaSO 4 (aq) -> Na2SO 4 (aq) + MgCO3 (s)
Ionic equation
CO32- (aq) + Ca2+ (aq) -> CaCO3 (s) CO32- (aq) + Mg2+ (aq) -> MgCO3 (s)
(iii)Adding calcium (II)Hydroxide/Lime water
Lime water/calcium Hydroxide removes only temporary Hardness of water from by precipitating insoluble calcium carbonate(IV).
chemical equation
Ca(OH)2 (aq) + Ca(HCO3) 2 (aq) -> 2H 2O(l) + 2CaCO3 (s)
Excess of Lime water/calcium Hydroxide sHould not be used because it dissolves again to form soluble calcium hydrogen carbonate(IV) causing the Hardness again.
(iv)Adding aqueous ammonia
Aqueous ammonia removes temporary Hardness of water by precipitating insoluble calcium carbonate(IV) and magnesium carbonate(IV)
chemical equation
2NH3 (aq) + Ca(HCO3) 2 (aq) -> (NH4) 2CO3(aq) + CaCO3 (s)
2NH3 (aq) + Mg(HCO3) 2 (aq) -> (NH4) 2CO3(aq) + MgCO3 (s)
(v)Use of ion-exChange permutit
thismethod involves packing a chamber with a resin made of insoluble complex of sodium salt called sodium permutit.
the sodium permutit releases sodium ions that are exchamber with Mg2+ and Ca2+ ions in Hard water making the water to be soft. i.e.
Na2X(aq) + Ca2+ (aq) -> Na+ (aq) + CaX(s)
Na2X(aq) + Mg2+ (aq) -> Na+ (aq) + MgX(s)
Hard water containing Mg2+ and Ca2+
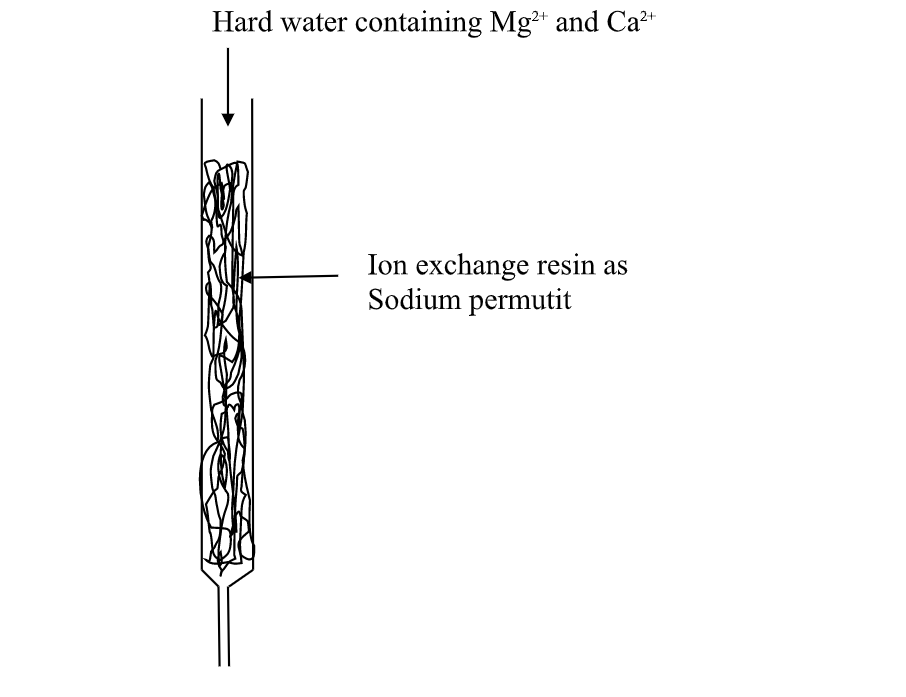
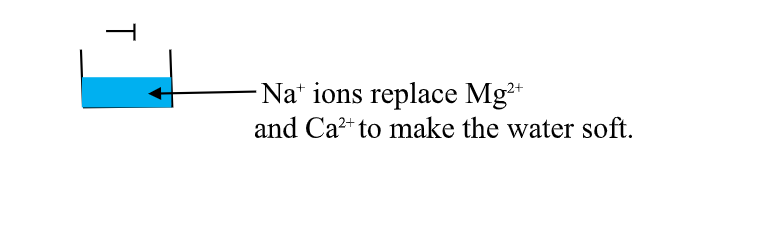
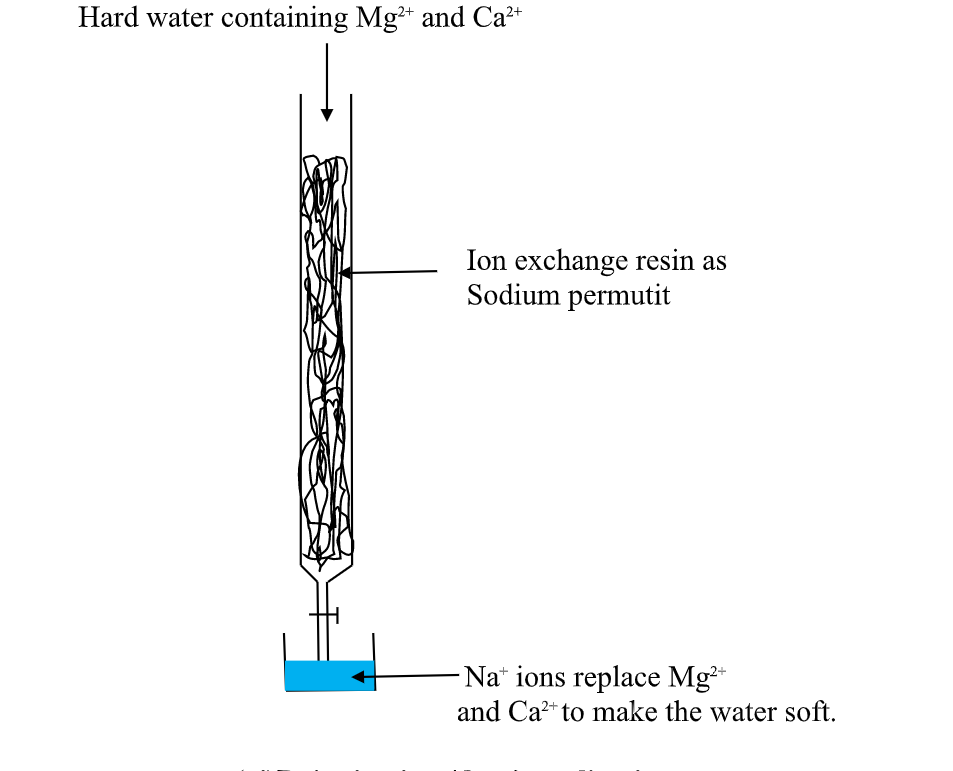
this is an advanced ion exChange method of producing deionized water .
Deionized water is extremely pure water made only of hydrogen and oxygen only without any dissolved substances. Deionization involve using the resins that remove all the cations by using:
(i)A cation exChanger which remove /absorb all the cations present in water and leave only H+ ions.
(ii)An anion exChanger which remove /absorb all the anions present in water and leave only OH- ions.
the H+(aq) and OH- (aq) neutralize eachother to form pure water.
chemical equation
H+(aq) + OH- (aq) -> H 2O(l) when exHausted the cation exChanger is regenerated by adding H+(aq) from Sulphuric(VI)acid/Hydrochloric acid.
when exHausted the anion exChanger is regenerated by adding OH-(aq) from sodium Hydroxide.
Advantages of Hard water
Hard water has the following advantages:
(i)Ca2+(aq) in Hard water are useful in bone and teetH formation
(ii) is good for brewing beer
(iii)contains minerals that cause it to havebetter /sweet taste
(iv)animals like snails and coral polyps use calcium to make their sHells and coral reefs respectively.
(v)processing mineral water
Disadvantages of Hard water
Hardness of water:
(i)waste a lot of soap during wasHing before lather is formed.
(ii)causes stains/blemisHes/marks on clothes/garments
(iii)causes fur on electric appliances like kettle ,boilers and pipes form decomposition of carbonates on heating .Thisreduces their efficiency Hence more/higher cost of power/electricity.
Sample revision questions
In an experiment, soap solution was added to three separate samples of water. the table below shows the volumes of soap solution required to form lather with 1000cm3 of eachsample of water before and after boiling.
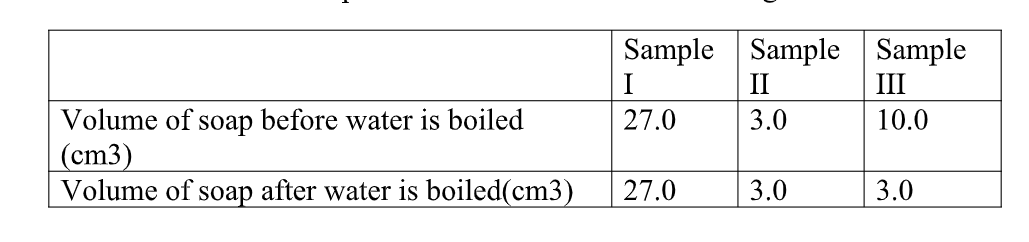
Sample II: Uses little sample of soap .
c) Name the Change in the volume of soap solution used in sample III (1mk)
On heating the sample water become soft bcause it is temporary Hard.
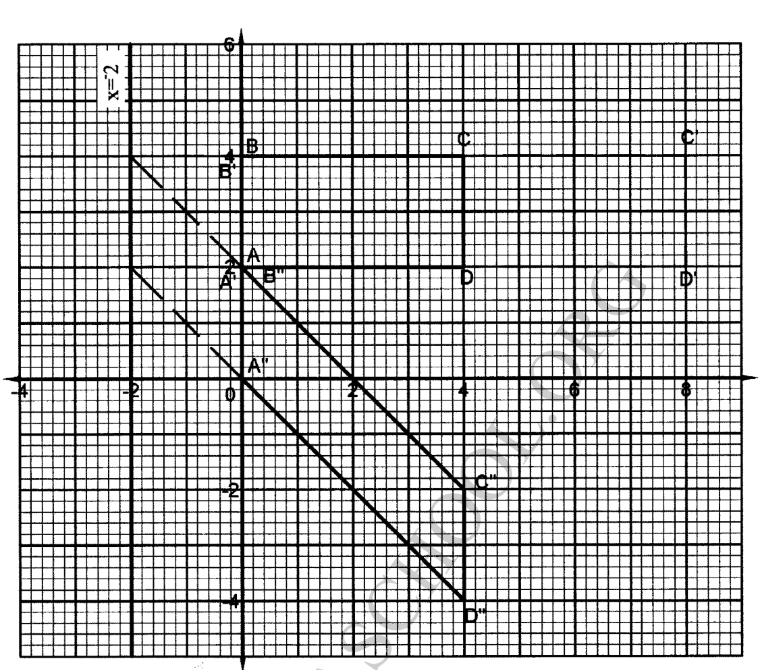
2.Study the scHeme below and use it to aanswer the questions that follow:
(i)Cation in solution K
Al3+
(ii)white ppt L
Al(OH)3
(iii) colourless solution M
[Al(OH)4]-
(iv) colourless solution N
AlCl3
(v)white ppt P
Al(OH)3
(b)Write the ionic equation for the reaction for the formation of:
(i)white ppt L
Al3+(aq) + 3OH- (aq) -> Al(OH)3(s)
(v)white ppt P
Al3+(aq) + 3OH- (aq) -> Al(OH)3(s)
(c)what property is illustrated in the formation of colourless solution M and N. amphotellic
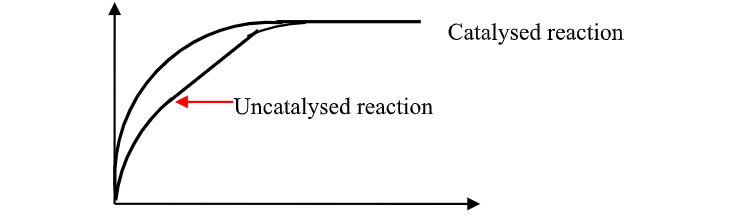
A.The Rate of chemical Reaction
(chemical Kinetics)
1.Introduction
the rate of a chemical reaction is the time taken for a given mass/amount of products to be formed.
the rate of a chemical reaction is also the time taken for a given mass/amount of reactant to be consumed /used up.
Some reactions are too slow to be determined. e.g rusting ,decomposition of hydrogen peroxide and weathering.
Some reactions are too fast and instantaneous e.g. neutralization of acid and bases/alkalis in aqueous solution and double decomposition/precipitation.
Other reactions are explosive and very risky to carry out safely e.g. reaction of potassium with water and sodium with dilute acids.
the study of the rate of chemical reaction is useful in knowing the factors that influence the reaction so that efficiency and profitability is maximized in industries.
theories of rates of reaction.
the rate of a chemical reaction is defined as the rate of Change of concentration/amount of reactants in unit time.
It is also the rate of formation of given concentration of products in unit time. i.e.
Rate of reaction = Change in concentration/amount of reactants
Time taken for the Change to occur
Rate of reaction = Change in concentration/amount of products formed
Time taken for the products to form
For the above, therefore the rate of a chemical reaction is rate of decreasing reactants to form an increasing product.
the SI unit of time is second(s) but minutes and Hours are also used.
(a)the collision theory
the collision theory is an application of the Kinetic theory of matter which assumes matter is made up of small/tiny/minute particles like ions atoms and molecules.
the collision theory proposes that
(i)for a reaction to occur, reacting particles must collide.
(ii)not all collisions between reacting particles are successful in a reaction. Collisions that initiate a chemical reaction are called successful / fruitful/ effective collisions
(iii)the speed at which particles collide is called collision frequency.
the higher the collision frequency the higher the cHances of successful / fruitful/ effective collisions to form products.
(iv)the higher the cHances of successful collisions, the faster the reaction.
(v)the average distance between solid particles from one another is too big for them to meet and collide successfully.
(vi)dissolving substances in a solvent ,make the solvent a medium for the reaction to take place.
the solute particle distance is reduced as the particle ions are free to move in the solvent medium.
(vii)successful collisions take place if the particles colliding havethe required energy and right orientation which increases their vibration and intensity of successful / fruitful/ effective collisions to form products.
(b)the Activation Energy(Ea) theory
the Enthalpy of activation(∆Ha) /Activation Energy(Ea) is the minimum amount of energy which the reactants must overcome before they react.
Activation Energy(Ea) is usually required /needed in bond breaking of the reacting particles.
Bond breaking is an endothermic process that require an energy input.
the higher the bond energy the slower the reaction to start of.
Activation energy does not influence whether a reaction is exothermic or endothermic.
the energy level diagrams below shows the activation energy for exothermic and endothermic processes/reactions.
Energy level diagram showing the activation energy for exothermic processes /reactions. Activated complex
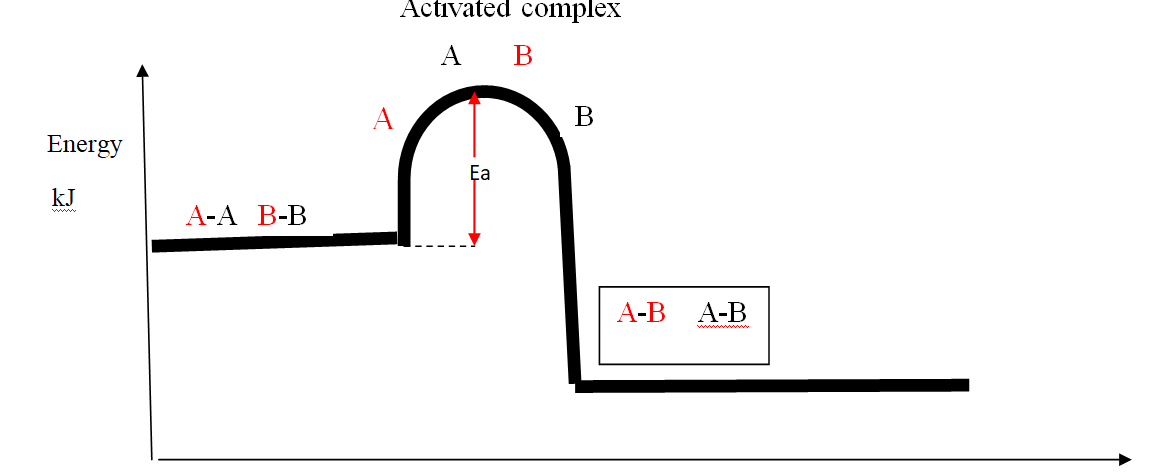
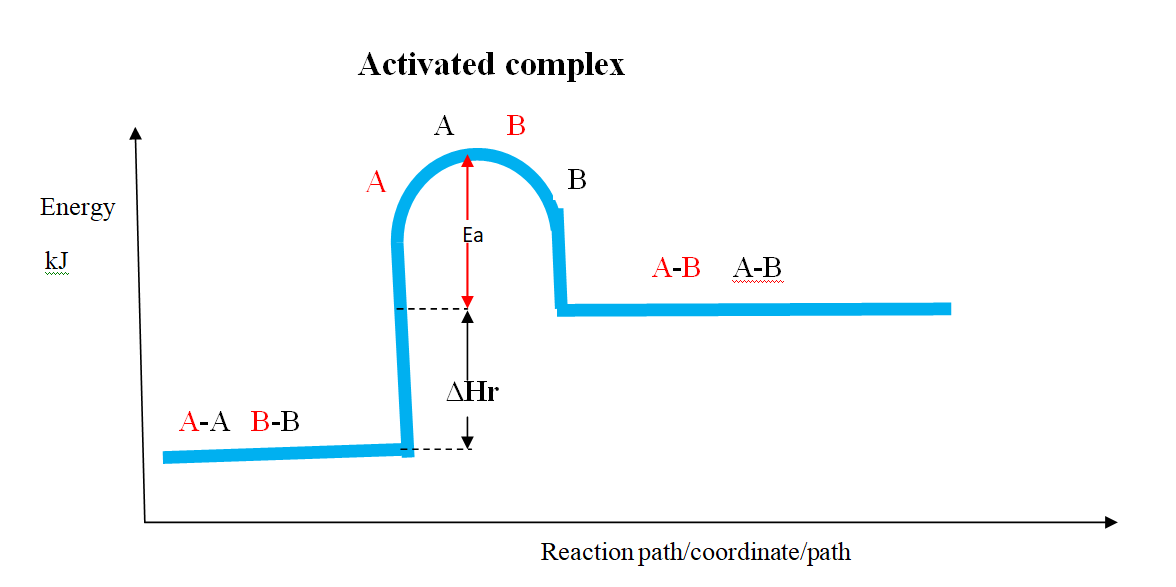
Exothermic reaction proceeds without further heating /external energy because it generates its own energy/Heat to overcome activation energy.
Endothermic reaction cannot proceed without further heating /external energy because it does not generates its own energy/Heat to overcome activation energy.
It generally therefore requires continuous supply of more energy/Heat to sustain it to completion.
3. Measuring the rate of a chemical reaction.
the rate of a chemical reaction can be measure as:
(i)Volume of a gas in unit time;
- if reaction is producing a gas as one of the products.
- if reaction is using a gas as one reactants
(ii)Change in mass of reactants/products for solid products/reactants in unit time.
(iii)formation of a given mass of precipitate in unit time
(iv)a certain mass of reactants to completely form products/diminisH.
Reactants may be Homogenous or Heterogenous.
-Homogenous reactions involve reactants in the same phase/state e.g. solid-solid,gas-gas,liquid-liquid.
-Heterogenous reactions involve reactants in the different phase/state e.g. solid-liquid,gas-liquid,solid-gas.
4. Factors influencing/altering/affecting/determining rate of reaction
the following factors alter/influence/affect/determine the rate of a chemical reaction:
(a)Concentration
(b)Pressure
(c) Temperature
(d)Surface area
(e)Catalyst
a) Influence of concentration on rate of reaction
the higher the concentration, the higher the rate of a chemical reaction. An increase in concentration of the reactants reduces the distance between the reacting particles increasing their collision frequency to form products.
Practically an increase in concentration reduces the time taken for the reaction to take place.
Practical determination of effect of concentration on reaction rate method 1(a)
Reaction of sodium thiSulphate with dilute Hydrochloric acid
Procedure:
Measure 20cm3 of 0.05M sodium thiSulphate into a 50cm3 glass beaker. Place the beaker on a white piece of filter paper with ink mark ‘X’ on it.
Measure 20cm3 of 0.1M Hydrochloric acid solution using a 50cm3 measuring cylinder.
Put the acid into the beaker containing sodium thiSulphate.
Immediately start off the stop watch/clock. Determine the time taken for the ink mark ‘X’ to become invisible /obscured when viewed from above.
Repeat the procedure by measuring different volumes of the acid and adding the volumes of the distilled water to complete table 1.
Sample results:Table 1.
Volume of acid(cm3) Volume of water(cm3) Volume of sodium thioSulphate(cm3) Time taken for mark ‘X’ to be invisible/obscured(seconds) Reciprocal of time
(a) complete table as evidence for all the practical work done and completed.
(b) (i)Consistent use of a decimal point on time as evidence of understanding/knowledge of the degree of accuracy of stop watches/clock.
(ii)Consistent use of a minimum of four decimal points on inverse/reciprocal of time as evidence of understanding/knowledge of the degree of accuracy of scientific calculator.
(c) accuracy against a school value based on candidate’s teachers-results submitted.
(d) correct trend (time increase as more water is added/acid is diluted) in conformity with expected theoretical results.
Sample questions
1. On separate graph papers plot a graph of:
(i)volume of acid used(x-axis) against time. Label thisgraph I
(ii) volume of acid used(x-axis) against 1/t. Label thisgraph II
2. Explain the shape of graph I
Diluting/adding water is causes a decrease in concentration.
Decrease in concentration reduces the rate of reaction by increasing the time taken for reacting particle to collide to form products.
sketch sample graph I
(i) 13cm3
From a correctly plotted graph
1/t at 13cm3 on the graph => 2.75 x 10-2
t = 1 / 2.75 x 10-2 = 36.3636 seconds
(ii) 15cm3
From a correctly plotted graph
1/t at 15cm3 on the graph => 3.35 x 10-2
t = 1 / 3.35 x 10-2 = 29.8507 seconds
(iii) 15cm3
From a correctly plotted graph
1/t at 17cm3 on the graph => 4.0 x 10-2
t = 1 / 4.0 x 10-2 = 25.0 seconds
(iv) 19cm3
From a correctly plotted graph
1/t at 19cm3 on the graph => 4.65 x 10-2
t = 1 / 4.65 x 10-2 = 21.5054 seconds
4.From graph II ,determine the volume of the acid used if the time taken for the cross to be obscured/invisible is:
(i)25 seconds
1/t => 1/25 = 4.0 x 10-2
Reading from a correctly plotted graph;
4.0 x 10-2 correspond to 17.0 cm3
(ii)30 seconds
1/t => 1/30 = 3.33 x 10-2
Reading from a correctly plotted graph;
3.33 x 10-2 correspond to 14.7 cm3
(iii)40 seconds
1/t => 1/40 = 2.5 x 10-2
Reading from a correctly plotted graph;
2.5 x 10-2 correspond to 12.3 cm3
4. Write the equation for the reaction taking place
Na2S2O3 (aq) + 2HCl(aq) -> 2NaCl (aq)+ SO (g) + S(s) + H2O(l)
Ionically:
S2O32- (aq) + 2H+ (aq) -> SO (g) + S(s) + H2O(l)
5.Name the yellow precipitate
Colloidal sulpHur
method 1(b)
Reaction of sodium tHiSulphate with dilute Hydrochloric acid
You are provided with
2.0M Hydrochloric acid
0.4M sodium tHioSulphate solution
Procedure:
Measure 10cm3 of sodium tHiSulphate into a 50cm3 glass beaker. Place the beaker on a white piece of filter paper with ink mark ‘X’ on it.
Add 5.0cm3 of Hydrochloric acid solution using a 10cm3 measuring cylinder into the beaker containing sodium tHiSulphate.
Immediately start off the stop watch/clock. Determine the time taken for the ink mark ‘X’ to become invisible /obscured when viewed from above.
Repeat the procedure by measuring different volumes of the tHioSulphate and adding the volumes of the distilled water to complete table 1.
Sample results:Table 1.
1. On separate graph papers plot a graph of:
(i)Concentration of sodium tHioSulphate against time. Label thisgraph I
(ii)Concentration of sodium tHioSulphate against against T-1.Label thisgraph II
2. Explain the shape of graph I
Diluting/adding water causes a decrease in concentration.
Decrease in concentration reduces the rate of reaction by increasing the time taken for reacting particle to collide to form products.
From graph II
Determine the time taken if
(i)12cm3 of sodium thiSulphate is diluted with 13cm3 of water. At 12cm3 concentration of sodium thiSulphate
= C1V1=C2V2 => 0.4 x 1 2 = C2x 25 =0.192M
From correct graph at concentration 0.192M => 2.4 x10-2
I/t = 2.4 x10-2 t = 41.6667seconds
(ii)22cm3 of sodium thiSulphate is diluted with 3cm3 of water.
At 22cm3 concentration of sodium thiSulphate
= C1V1=C2V2 => 0.4 x 22 = C2x 25 =0.352M
From correct graph at concentration 0.352M => 3.6 x10-2
I/t = 3.6 x10-2 t = 27.7778seconds
Determine the volume of water and sodium tHioSulphate if T-1 is 3.0 x10-1
From correct graph at T-1 = 3.0 x10-1 => concentration = 0.65 M
= C1V1=C2V2 => 0.4 x 25 = 0.65 M x V2 = 15.3846cm3
Volume of water = 25 - 15.3846cm3 = 9.6154cm3
Determine the concentration of Hydrochloric acid if 12cm3 of sodium tHioSulphate and 13cm3 of water was used.
At 12cm3 concentration of sodium tHiSulphate
= C1V1=C2V2 => 0.4 x 1 2 = C2x 25 =0.192M
Mole ratio Na2S2 O3 :HCl =1:2
Moles of Na2S2 O3 = 0.192M x 12 => 2.304 x 10-3 moles
1000 Mole ratio HCl =2.304 x 10-1 moles = 1.152 x 10-3 moles
2 Molarity o f HCl = 1.152 x 10-3 moles x 1000 = 0.2304M
5.0
method 2
Reaction of Magnesium with dilute Hydrochloric acid
Procedure
Scub 10centimeter lengthof magnesium ribbon with sand paper/steel wool.
Measure 40cm3 of 0.5M dilute Hydrochloric acid into a flask .
Fill a graduated gas jar with water and invert it into a trougH. Stopper the flask and set up the apparatus to collect the gas produced as in the set up below:
Carefully remove the stopper, carefully put the magnesium ribbon into the flask . cork tigHtly.
Add the acid into the flask. Connect the delivery tube into the gas jar.
Immediately start off the stop watch and determine the volume of the gas produced after every 30 seconds to complete table II below.
Sample results: Table II
1.Plot a graph of volume of gas produced (y-axis) against time
the rate of reaction is faster when the concentration of the acid is High .
As time goes on, the concentration of the acid decreases and therefore less gas is produced.
when all the acid has reacted, no more gas is produced after 210 seconds and the graph flattens.
3.Calculate the rate of reaction at 120 seconds
From a tangent at 120 seconds rate of reaction = Change in volume of gas Change in time => From the tangent at 120seconds V2 - V1 = 96-84 = 12 = 0.2cm3sec-1 T2 - T1 150-90 60
4. Write an ionic equation for the reaction taking place.
Mg2+(s) + 2H+(aq) -> Mg2+(aq) + H2 (g)
5. On the same axis sketch then explain the curve that would be obtained if:
(i) 0.1 M Hydrochloric acid is used –Label thiscurve I
(ii)1.0 M Hydrochloric acid is used –Label thiscurve II
Observation:
Curve I is to the right
Curve II is to the left
Explanation
A decrease in concentration shift the rate of reaction graph to the right as more time is taken for completion of the reaction.
An increase in concentration shift the rate of reaction graph to the left as less time is taken for completion of the reaction.
both graphs flatten after some time indicating the completion of the reaction.
b)Influence of pressure on rate of reaction
Pressure affects only gaseous reactants.
An increase in pressure reduces the volume(Boyles law) in which the particles are contained. Decrease in volume of the container bring the reacting particles closer to eachother which increases their cHances of effective/successful/fruitful collision to form products.
An increase in pressure therefore increases the rate of reaction by reducing the time for reacting particles of gases to react.
At industrial level, the following are some reactions that are affected by pressure: (a)Haber process for manufacture of ammonia
(b)Contact process for manufacture of Sulphuric(VI)acid
(c)Ostwalds process for the manufacture of nitric(V)acid
the influence of pressure on reaction rate is not felt in solids and liquids.
this is because the solid and liquid particles havefixed positions in their strong bonds and therefore no degree of freedom (Kinetic theory of matter)
c)Influence of temperature on rate of reaction
An increase in temperature increases the kinetic energy of the reacting particles by increasing their collision frequency.
Increase in temperature increases the particles which can overcome the activation energy (Ea).
A 10oC rise in temperature doubles the rate of reaction by reducing the time taken for the reaction to complete by a half.
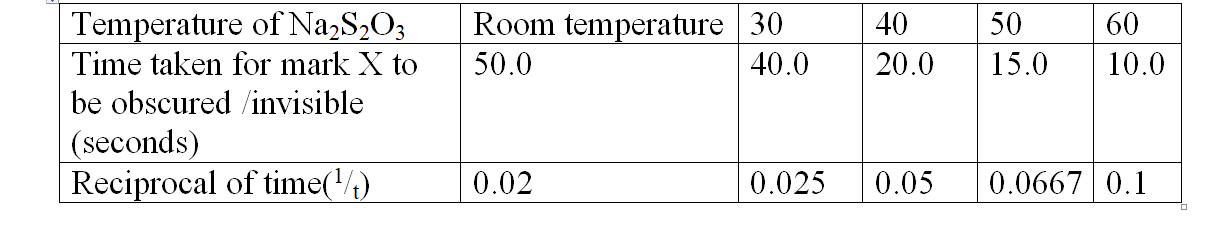
Practical determination of effect of Temperature on reaction rate
method 1
Reaction of sodium tHiSulphate with dilute Hydrochloric acid
Procedure:
Measure 20cm3 of 0.05M sodium tHiSulphate into a 50cm3 glass beaker. Place the beaker on a white piece of filter paper with ink mark ‘X’ on it. Determine and record its temperature as room temperature in table 2 below. Measure 20cm3 of 0.1M Hydrochloric acid solution using a 50cm3 measuring cylinder.
Put the acid into the beaker containing sodium tHiSulphate.
Immediately start off the stop watch/clock.
Determine the time taken for the ink mark ‘X’ to become invisible /obscured when viewed from above.
Measure another 20cm3 separate portion of the tHiSulphate into a beaker, Heat the solution to 30oc.
Add the acid into the beaker and repeat the procedure above. Complete table 2 below using different temperatures of the tHioSulphate.
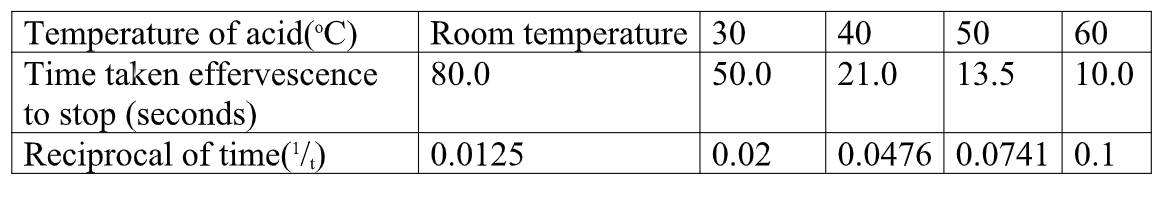
Sample results:Table 2.

1. Plot a graph of temperature(x-axis) against 1/t

(i)1/t is ;
I. 0.03
Reading directly from a correctly plotted graph = 32.25 oc II. 0.07
Reading directly from a correctly plotted graph = 48.0 oc (ii) t is;
I. 30 seconds
30 seconds => 1/t =1/30 =0.033
Reading directly from a correctly plotted graph 0.033 => 33.5 oc
II. 45 seconds
45 seconds => 1/t =1/45 =0.022
Reading directly from a correctly plotted graph 0.022 => 29.0 oc
III. 25 seconds
25 seconds => 1/t =1/25 =0.04
Reading directly from a correctly plotted graph 0.04 => 36.0 oc
(b) From your graph determine the time taken for the cross to become invisible at:
(i) 57.5 oc
Reading directly from a correctly plotted graph at 57.5 oc= 0.094
=>1/t = 0.094
t= 1/0.094 => 10.6383 seconds
(ii) 45 oc
Reading directly from a correctly plotted graph at 45 oc = 0.062
=>1/t = 0.062
t= 1/0.094 => 16.1290 seconds
(iii) 35 oc
Reading directly from a correctly plotted graph at 35 oc = 0.047
=>1/t = 0.047
t= 1/0.047 => 21.2766 seconds
method 2
Reaction of Magnesium with dilute Hydrochloric acid
Procedure
Scub 5centimeter lengthof magnesium ribbon with sand paper/steel wool. Cut the piece into five equal one centimeter smaller pieces.
Measure 20cm3 of 1.0M dilute Hydrochloric acid into a glass beaker .
Put one piece of the magnesium ribbon into the acid, swirl.
Immediately start off the stop watch/clock.
Determine the time taken for the effervescence/fizzing/bubbling to stop when viewed from above.
Record the time in table 2 at room temperature.
Measure another 20cm3 portions of 1.0M dilute Hydrochloric acid into a clean beaker.
Heat separately one portion to 30oc, 40oc , 50oC and 60oc and adding 1cm lengthof the ribbon and determine the time taken for effervescence /fizzing /bubbling to stop when viewed from above .
Record eachtime to complete table 2 below using different temperatures of the acid.
Sample results:Table 1.
1. Plot a graph of temperature(x-axis) against 1/t
Moles = Mass of magnesium => 1.0 = 4.167 x 10 -2 moles
Molar mass of Mg 24
(b)Calculate the number of moles of Hydrochloric acid used
Moles of acid = molarity x volume of acid
1000
=> 1.0 x 20 = 2.0 x 10 -2 moles 1000
(c)Calculate the mass of magnesium that remain unreacted
Mole ratio Mg: HCl = 1:2
Moles Mg = ½ moles HCl
=> ½ x 2.0 x 10 -2 moles = 1.0 x 10 -2 moles
Mass of reacted Mg = moles x molar mass
=> 1.0 x 10 -2 moles x 24 = 0.24 g
Mass of unreacted Mg = Original total mass - Mass of reacted Mg
=> 1.0 g – 0.24 = 0.76 g
(b)Calculate the total volume of hydrogen gas produced during the above reactions.
Mole ratio Mg : H2 = 1:1
Moles of Mg that reacted per experiment = moles H2 =1.0 x 10 -2 moles
Volume of hydrogen at s.T.p produced per experiment = moles x 24 dm3
=> 1.0 x 10 -2 moles x 24 dm3 = 0.24dm3
Volume of hydrogen at s.T.p produced in 5 experiments =0.24 dm3 x 5 = 1.2 dm3
3.(a)At what temperature was the time taken for magnesium to react equal to:
(i)70seconds
70 seconds => 1/t =1/70 =0.01429
Reading directly from a correctly plotted graph 0.01429 => 28.0 oc
(ii)40seconds
40 seconds => 1/t =1/40 =0.025
Reading directly from a correctly plotted graph 0.025 => 32.0 oc
(b)what is the time taken for magnesium to react if the reaction was done at:
(i) 55.0 oc
Reading directly from a correctly plotted graph at 55.0 oc=> 1/t = 8.0 x 10-2
=> t = 1/8.0 x 10-2 = 12.5 seconds
(ii) 47.0 oc
Reading directly from a correctly plotted graph at 47.0 oc=> 1/t = 6.0 x 10-2
=> t = 1/6.0 x 10-2 = 16.6667 seconds
(iii) 33.0 oc
Reading directly from a correctly plotted graph at 33.0 oc=> 1/t = 2.7 x 10-2
=> t = 1/2.7 x 10-2 = 37.037 seconds
4. Explain the shape of the graph.
Increase in temperature increases the rate of reaction as particles gain kinetic energy increasing their frequency and intensity of collision to form products.
d)Influence of surface area on rate of reaction
Surface area is the area of contact. An increase in surface area is a decrease in particle size.
Practically an increase in surface area involves cHopping /cutting solid lumps into smaller pieces/chips then crusHing the chips into powder.
chips thus havea higher surface area than solid lumps but powder has a Highest surface area.
An increase in surface area of solids increases the area of contact with a liquid solution increasing the cHances of successful/effective/fruitful collision to form products.
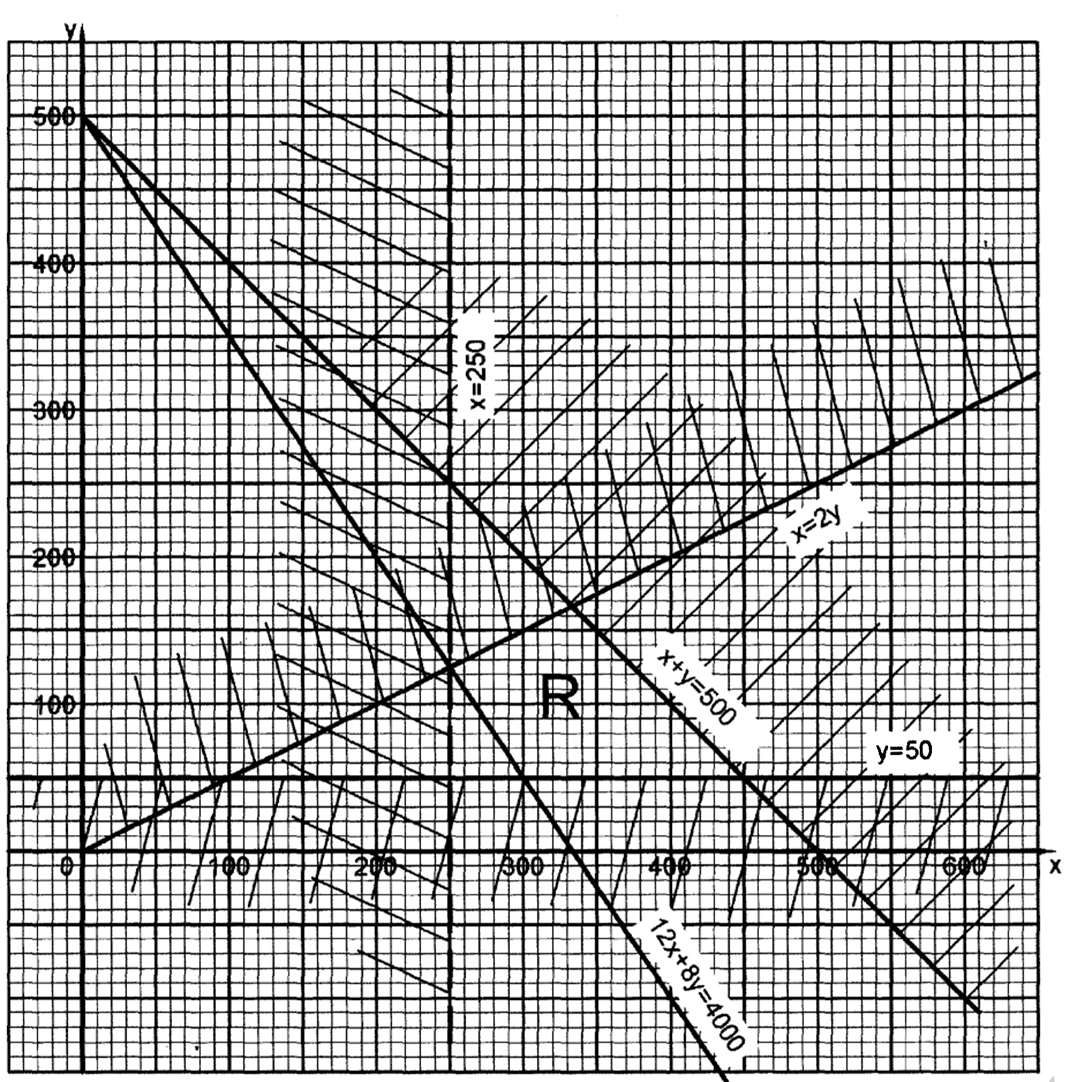
the influence of surface area on rate of reaction is mainly in Heterogeneous reactions. Reaction of cHalk/calcium carbonate on dilute Hydrochloric acid
Procedure
Measure 20cm3 of 1.0 M Hydrochloric acid into three separate conical flasks labeled C1 C2 and C3 .
Using a watch glass Weigh three separate 2.5g a piece of white cHalk. Place the conical flask C1 on an electronic balance.
Reset the balance scale to 0.0.
Put one Weighed sample of the cHalk into the acid in the conical flask. Determine the scale reading and record it at time =0.0.
Simultaneously start of the stop watch.
Determine and record the scale reading after every 30 seconds to complete Table I .
Repeat all the above procedure separately with C2 and C3 to complete Table II and Table III by cutting the cHalk into small pieces/chips for C2 and crusHing the cHalk to powder for C3
Sample results:Table 1.
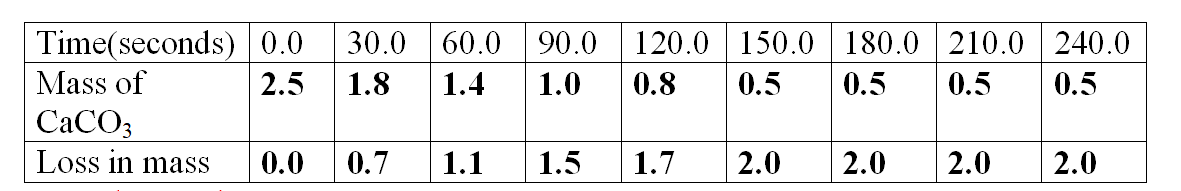
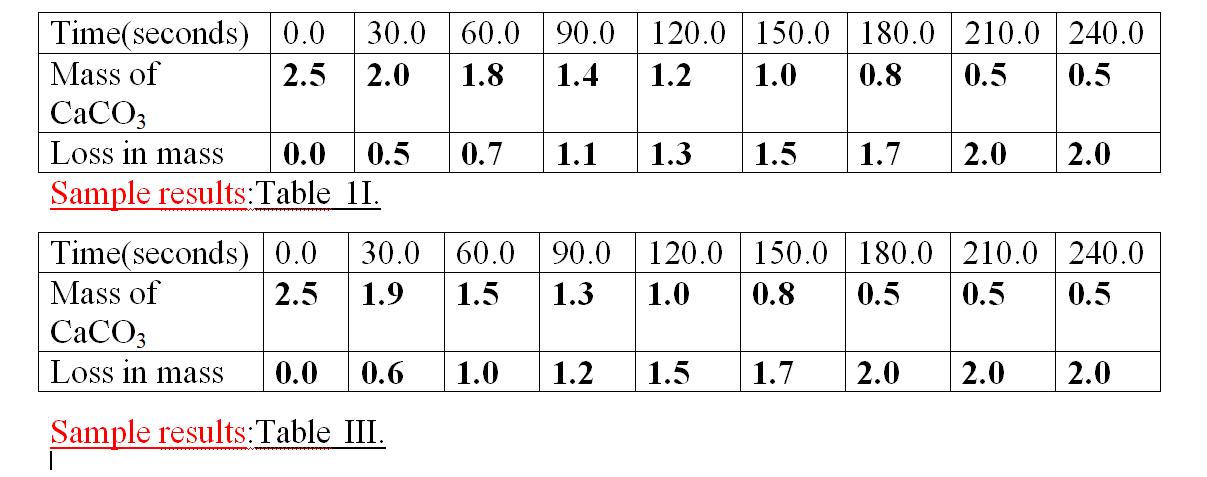
1.Calculate the loss in mass made at the end of eachtime from the original to complete table I,II and III
2.On the same axes plot a graph of total loss in mass against time (x-axes) and label them curve I, II, and III from Table I, II, and III.
3.Explain why there is a loss in mass in all experiments.
Calcium carbonate react with the acid to form carbon(IV)oxide gas that escape to the atmosphere.
4.Write an ionic equation for the reaction that take place
CaCO3(s) + 2H+(aq) -> Ca2+(aq) + H2O(l) + CO(g)
5.Sulphuric(VI)acid cannot be used in the above reaction.
On the same axes sketch the curve which would be obtained if the reaction was attempted by reacting a piece of a lump of cHalk with 0.5M Sulphuric(VI)acid. Label it curve IV.
Explain the shape of curve IV.
Calcium carbonate would react with dilute 0.5M Sulphuric(VI)acid to form insoluble calcium Sulphate(VI) that coat /cover unreacted Calcium carbonate stopping the reaction from reaching completion.
6.Calculate the volume of carbon(IV)oxide evolved(molar gas volume at room temperature = 24 dm3, C= 12.0, O= 16.O Ca=40.0)
method I
Mole ratio CaCO3(s) : CO(g) = 1:1
Moles CaCO3(s) used = Mass CaCO3(s) = 0.025 moles
Molar mass CaCO3(s)
Moles CO(g) = 0.025 moles
Volume of CO(g) = moles x molar gas volume
=>0.025 moles x 24 dm3 = 0.600 dm3/600cm3
method II
Molar mass of CaCO3(s) = 100g produce 24 dm3 of CO(g)
Mass of CaCO3(s) =2.5 g produce 2.5 x 24 = 0.600dm3
100 7.From curve I ,determine the rate of reaction (loss in mass per second)at time 180 seconds on the curve.
From tangent at 180 seconds on curve I
Rate = M2-M1 => 2.08 – 1.375 = 0.625 = 0.006944g sec-1 T2- T1 222-132
90 8.what is the effect of particle size on the rate of reaction?
A larger surface area is a reduction in particle size which increases the area of contact between reacting particles increasing their collision frequency.
theoretical examples
1. Excess marble chips were put in a beaker containing 100cm3 of 0.2M Hydrochloric acid.
the beaker was then placed on a balance and total loss in mass recorded after every two minutes as in the table below.
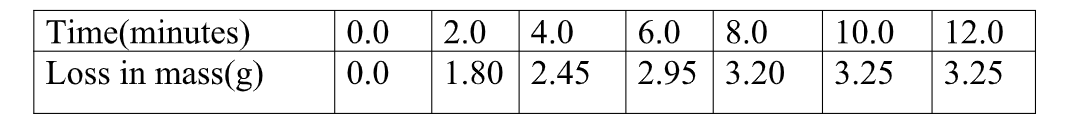
Carbon (IV) oxide gas was produced that escape to the surrounding
(b)Calculate the average rate of loss in mass between:
(i) 0 to 2 minutes Average rate =M2-M1 => 1.80 – 0.0 = 1.8 = 9.00g min-1
T2- T1 2.0 – 0.0 2 (i) 6 to 8 minutes Average rate =M2-M1 => 3.20 – 2.95 = 0.25 = 0.125g min-1
T2- T1 8.0 – 6.0 2
(iii) Explain the difference between the average rates of reaction in (i) and(ii) above.
Between 0 and 2 minutes , the concentration of marble chips and Hydrochloric acid is High therefore there is a higher collision frequency between the reacting particles leading to High successful rate of formation of products.
Between 6 and 8 minutes , the concentration of marble chips and Hydrochloric acid is low therefore there is low collision frequency between the reacting particles leading to less successful rate of formation of products.
(c)Write the equation for the reaction that takes place.
CaCO3(s) + 2HCl (aq) -> CaCO3 (aq) + H2O(l) + CO(g)
(d)State and explain three ways in which the rate of reaction could be increased.
(i)heating the acid- increasing the temperature of the reacting particles increases their kinetic energy and thus collision frequency.
(ii)Increasing the concentration of the acid-increasing in concentration reduces the distances between the reacting particles increasing their cHances of effective/fruitful/successful collision to form products faster.
(iii)CrusHing the marble chips to powder- Thisreduces the particle size/increase surface area increasing the area of contact between reacting particles.
(e)If the solution in the beaker was evaporated to dryness then left overnigHt in the open, explain what would Happen.
It becomes wet because calcium (II) chloride absorbs water from the atmosphere and form solution/is deliquescent.
(f)when sodium Sulphate (VI) was added to a portion of the contents in the beaker after the reaction , a white precipitate was formed .
(i)Name the white precipitate.
Calcium(II)Sulphate(VI)
(ii)Write an ionic equation for the formation of the white precipitate
Ca2+(aq) + SO42-(aq)->CaSO4(s)
(iii)State one use of the white precipitate
-Making plaster for building
-Manufacture of plaster of Paris
-Making Sulphuric(VI)acid
(g)(i) Plot a graph of total loss in mass(y-axes) against time (ii)From the graph, determine the rate of reaction at time 2 minutes.
From a tangent/slope at 2 minutes;
Rate of reaction = Average rate =M2-M1 => 2.25 – 1.30 = 0.95 = 0.3958g min-1 T2- T1 3.20 – 0.8 2.4 (iii)sketch on the same axes the graph that would be obtained if 0.02M Hydrochloric acid was used. Label it curve II
e) Influence of catalyst on rate of reaction
Catalyst is a substance that alter the rate /speed of a chemical reaction but remain chemically unchamber at the end of a reaction. Biological catalysts are called enzymes.
A catalyst does not alter the amount of products formed but itself may be altered physically e.g. from solid to powder to fine powder.
Like biological enzymes, a catalyst only catalyse specific type of reactions
Most industrial catalysts are transition metals or their compounds. Catalyst works by lowering the Enthalpy of activation(∆Ha)/activation energy (Ea) of the reactants .
The catalyst lowers the Enthalpy of activation(∆Ha)/activation energy (Ea) by:
(i) forming sHort lived intermediate compounds called activated complex that break up to form the final product/s
(ii) being absorbed by the reactants thus providing the surface area on which reaction occurs.
A catalyst has no effect on the Enthalpy of reaction ∆Hr but only lowers the Enthalpy of activation(∆Ha)/activation energy (Ea)It thus do not affect/influence whether the reaction is exothermic or endothermic as shown in the energy level diagrams below.
Energy level diagram showing the activation energy for exothermic processes /reactions.
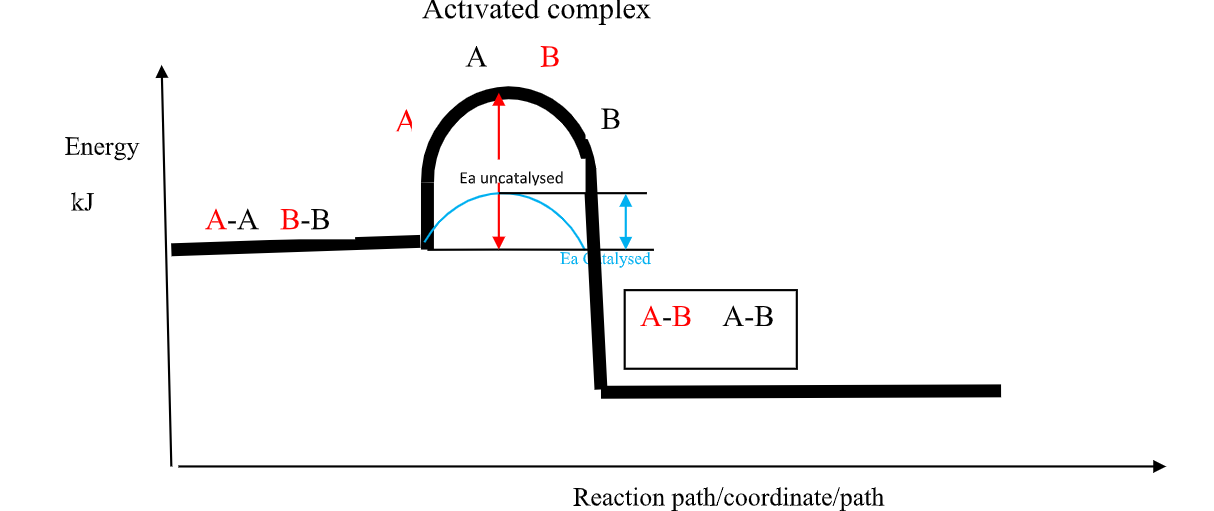
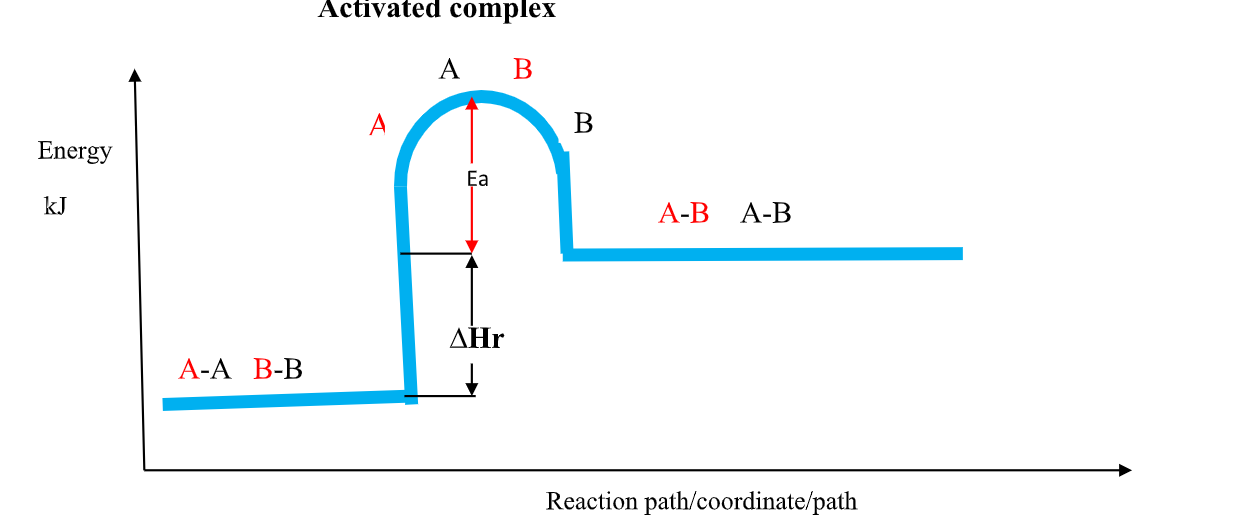
(a)the contact process
Vanadium(V) Oxide(V2O5) or platinum(Pt) catalyses the oxidation of sulpHur(IV)oxide during the manufacture of Sulphuric(VI) acid from contact process.
SO(g) + O(g) ----V2O5--> SO3(g)
To reduce industrial cost of manufacture of Sulphuric (VI) acid from contact process Vanadium(V) Oxide(V2O5) is used because it is cHeaper tHougH it is easily poisoned by impurities.
(b)Ostwalds process
Platinum promoted with Rhodium catalyses the oxidation of ammonia to nitrogen(II)oxide and water during the manufacture of nitric(V)acid
4NH3(g) + 5O(g) ---- Pt/RH--> 4NO (g) + 6H2O(l)
(c)Haber process
Platinum or iron catalyses the combination of nitrogen and hydrogen to form ammonia gas N2(g) + 3H2(g) --- Pt or Fe---> 2NH3(g)
(d)hydrogenation/Hardening of oil to fat
Nickel (Ni) catalyses the hydrogenation of unsaturated compound containing
- C=C- or –C=C- to saturated compounds without double or triple bond thisprocess is used is used in Hardening oil to fat.
(e)Decomposition of hydrogen peroxide
Manganese(IV)oxide speeds up the rate of decomposition of hydrogen peroxide to water and oxygen gas.
thisprocess/reaction is used in the school laboratory preparation of Oxygen.
2H2O (g) ----MnO--> O(g) + 2H2O(l)
(f)Reaction of metals with dilute Sulphuric(VI)acid
Copper(II)Sulphate(VI) speeds up the rate of production of hydrogen gas from the reaction of Zinc and dilute Sulphuric(VI)acid.
thisprocess/reaction is used in the school laboratory preparation of hydrogen.
H2 SO4 (aq) + Zn(s) ----CuSO4--> ZnSO4 (aq) + H2(g)
(g) Substitution reactions
when placed in bright sunlight or U.V /ultraviolet light , a mixture of a Halogen and an alkane undergo substitution reactions explosively to form Halogenoalkanes. when paced in diffused sunlight the reaction is very slow.
e.g. CH4(g) + Cl2(g) ---u.v. light--> CHCl(g) + HCl(g)
(H)Photosynthesis
Plants convert carbon(IV)oxide gas from the atmosphere and water from the soil to form glucose and oxygen as a byproduct using sunlight / ultraviolet light.
(i)Photography
Photographic film contains silver bromide emulsion which decomposes to silver and bromine on exposure to sunlight.
2AgBr(s) ---u.v/sun light--> 2Ag(s) + Br2(l)
when developed, the silver deposits give the picture of the object whose photograph was taken depending on intensity of light.
A picture photographed in diffused light is therefore blurred.
Practical determination of effect of catalyst on decomposition of hydrogen peroxide
Measure 5cm3 of 20 volume hydrogen peroxide and then dilute to make 40cm3 in a measuring cylinder by adding distilled water.
Divide it into two equal portions.
(i)Transfer one 20cm3volume hydrogen peroxide into a conical/round bottomed/flat bottomed flask.
Cork and swirl for 2 minutes. Remove the cork.
Test the gas produced using a glowing splint. Clean the conical/round bottomed/flat bottomed flask.
(ii)Put 2.0g of Manganese (IV) oxide into the clean conical/round bottomed/flat bottomed flask. Stopper the flask.
Transfer the second portion of the 20cm3volume hydrogen peroxide into a conical/round bottomed/flat bottomed flask throughthe dropping/thistle funnel.
Connect the delivery tube to a calibrated/graduated gas jar as in the set up below.
Start off the stop watch and determine the volume of gas in the calibrated/graduated gas jar after every 30 seconds to complete Table 1.
(iii)Weigh a filter paper .Use the filter paper to filter the contents of the conical conical/round bottomed/flat bottomed flask.
Put the residue on a sand bathto dry.
Weigh the dry filter paper again .Determine the new mass Manganese (IV) oxide.
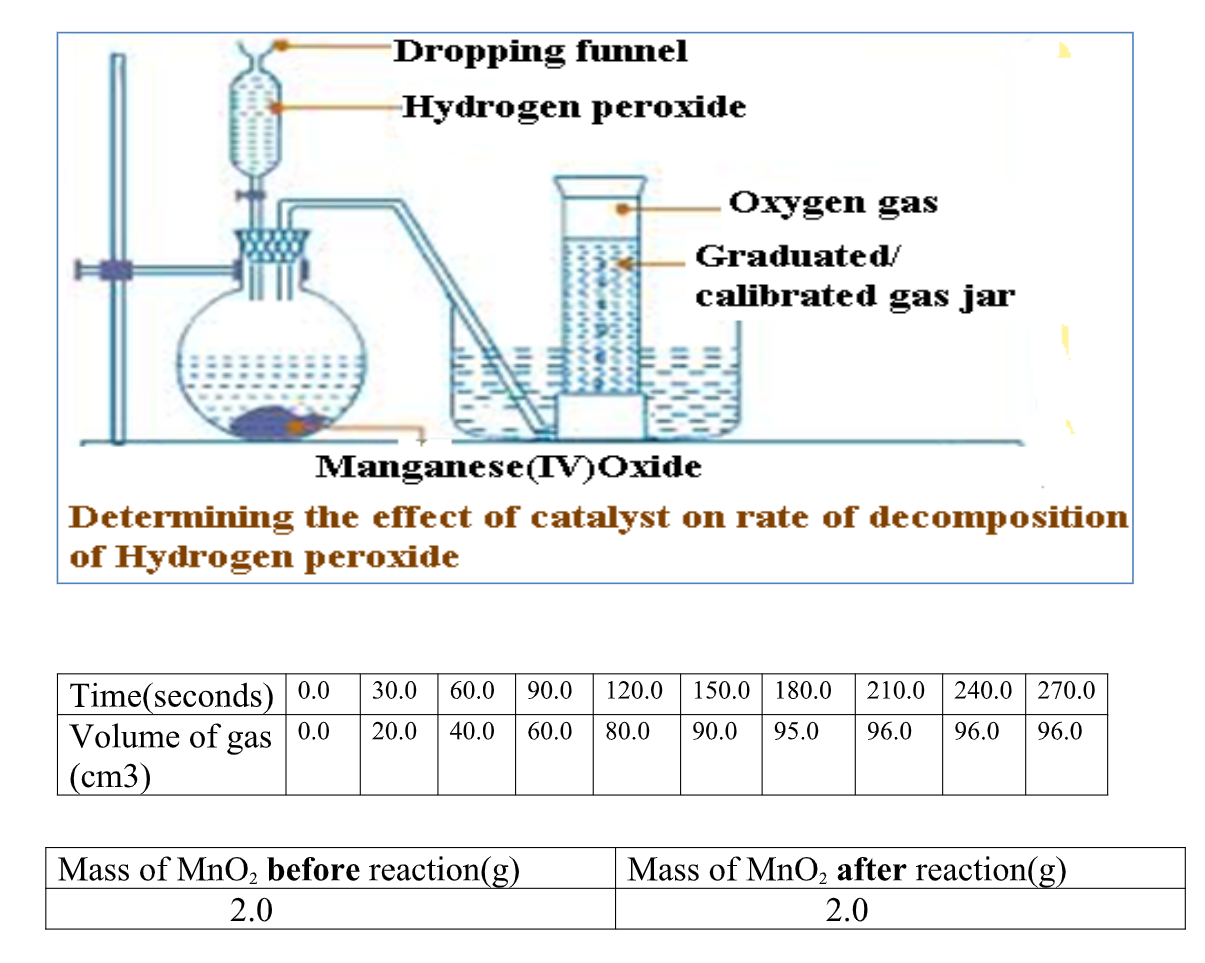

(c) Explain the changes in mass of manganese(IV)oxide before and after the reaction.
the mass of MnO before and after the reaction is the same but a more fine powder after the experiment. A catalyst therefore remains unchamber chemically but may physically Change.
B.equilibria (chemical Cybernetics)
Equilibrium is a state of balance.
chemical equilibrium is state of balance between the reactants and products.
As reactants form products, some products form back the reactants.
Reactions in which the reactants form products to completion are said to be reversible i.e. A + B -> C + D
Reactions in which the reactants form products and the products can reform the reactants are said to be reversible.
A + B C + D
Reversible reactions may be:
(a)Reversible physical changes
(b)Reversible chemical changes
(c)Dynamic equilibrium
(a)Reversible physical changes
Reversible physical Change is one which involves:
(i) Change of state/phase from solid, liquid, gas or aqueous solutions.
States of matter are interconvertible and a reaction involving a Change from one state/phase can be reversed back to the original.
(ii) colour changes. Some substances/compounds Change their colours without Change in chemical substance.
Examples of reversible physical changes
(i) colour Change on heating and cooling:
I. Zinc(II)Oxide changes from white when cool/cold to yellow when hot/heated and back.
ZnO(s) ZnO(s)
(white when cold) (yellow when hot)
II. Lead(II)Oxide changes from yellow when cold/cool to brown when hot/heated and back.
PbO(s) PbO(s)
(brown when hot) (yellow when cold)
(ii)Sublimation
I. Iodine sublimes from a grey crystalline solid on heating to purple vapour. Purple vapour undergoes deposition back to the grey crystalline solid.
I2(s) I2(g)
(grey crystalline solid (purple vapour
undergo sublimation) undergo deposition)
II. Carbon (IV)oxide gas undergoes deposition from a colourless gas to a white solid at very High pressures in a cylinder.
It sublimes back to the colourless gas if pressure is reduced CO(s) CO(g)
(white powdery solid (colourless/odourless gas
undergo sublimation) undergo deposition)
(iii)Melting/ freezing and boiling/condensation
Ice on heating undergo melting to form a liquid/water. Liquid/water on further heating boil/vaporizes to form gas/water vapour.
Gas/water vapour on cooling, condenses/liquidifies to water/liquid. On further cooling, liquid water freezes to ice/solid.
Melting boiling
Freezing condensing
(iv)Dissolving/ crystallization/distillation
Solid crystals of soluble substances (solutes) dissolve in water /solvents to form a uniform mixture of the solute and solvent/solution.
On crystallization /distillation /evaporation the solvent evaporate leaving a solute back. e.g.
NaCl(s) + aq NaCl(aq)
(b)Reversible chemical changes
these are reactions that involve a chemical Change of the reactants which can be reversed back by recombining the new substance formed/products.
Examples of Reversible chemical changes
(i)heating Hydrated salts/adding water to anhydrous salts.
when Hydrated salts are heated they lose some/all their water of crystallization and become anhydrous.
heating an unknown substance /compound that forms a colourless liquid droplets on the cooler parts of a dry test/boiling tube is in fact a confirmation inference that the substance/compound being heated is Hydrated.
when anhydrous salts are added (back) some water they form Hydrated compound/salts.
heating Copper(II)Sulphate(VI)pentahydrate and cobalt(II)chloride Hexahydrate
(i)Heat about 5.0g of Copper(II)Sulphate(VI) pentahydrate in a clean dry test tube until there is no further colour Change on a small Bunsen flame. Observe any changes on the side of the test/boiling tube.
Allow the boiling tube to cool.Add about 10 drops of distilled water. Observe any changes.
(ii)Dip a filter paper in a solution of cobalt(II)chloride Hexahydrate.
Pass one end the filter paper to a small Bunsen flame repeatedly. Observe any changes on the filter paper.
Dip the paper in a beaker containing distilled water. Observe any changes.
Sample observations
Hydrated compound Observation before heating Observation after heating
Observation on adding water
Copper(II)Sulphate
(VI) pentahydrate Blue crystalline solid (i)colour changes from blue to white.
(ii)colourless liquid forms on the cooler parts of boiling / test tube (i)colour changes from white to blue
(ii)boiling tube becomes warm /hot.
Cobalt(II)chloride Hexahydrate Pink crystalline solid/solution (i)colour changes from pink to blue.
(ii) colourless liquid forms on the cooler parts of boiling / test tube (if crystal are used) (i)colour changes from blue to pink (ii)boiling tube becomes warm/hot.
when blue Copper(II)Sulphate (VI) pentahydrate is heated, it loses the five molecules of water of crystallization to form white anhydrous Copper(II)Sulphate (VI).
Water of crystallization form and condenses as colourless droplets on the cooler parts of a dry boiling/test tube.
this is a chemical Change that produces a new substance. On adding drops of water to an anhydrous white copper(II)Sulphate(VI) the Hydrated compound is formed back.
the Change from Hydrated to anhydrous and back is therefore reversible chemical Change.
both anhydrous white copper(II)Sulphate(VI) and blue cobalt(II)chloride Hexahydrate are therefore used to test for the presence of water when they turn to blue and pink respectively.
(white/anhydrous) (blue/Hydrated)
(blue/anhydrous) (pink/Hydrated)
(ii)chemical sublimation
Some compounds sublime from solid to gas by dissociating into new different compounds. e.g.
heating ammonium chloride
(i)Dip a glass rod containing concentrated Hydrochloric acid. Bring it near the mouth of a bottle containing concentrated ammonia solution.
Explain the observations made.
when a glass rod containing hydrogen chloride gas is placed near ammonia gas, they react to form ammonium chloride solid that appear as white fumes.
thisexperiment is used interChangeably to test for the presence of hydrogen chloride gas (and Hence Cl- ions) and ammonia gas (and Hence NH4+ ions)
(ii)Put 2.0 g of ammonium chloride in a long dry boiling tube. Place wet / moist /damp blue and red litmus papers separately on the sides of the mouth of the boiling tube.
Heat the boiling tube gently then strongly.
Explain the observations made.
when ammonium chloride is heated it dissociates into ammonia and hydrogen chloride gases.
Since ammonia is less dense, it diffuses faster to turn both litmus papers blue before hydrogen chloride turn red because it is denser.
the heating and cooling of ammonium chloride is therefore a reversible chemical Change.
(Turns moist (Turns moist (forms white fumes)
litmus paper blue) litmus paper red)
(c)Dynamic equilibria
For reversible reactions in a closed system:
(i) at the beginning;
- The reactants are decreasing in concentration with time
- The products are increasing in concentration with time
(ii) after some time a point is reached when as the reactants are forming products the products are forming reactants. this is called equilibrium.
(i) a reaction from left to right (reactants to products) is called forward reaction.
(ii) a reaction from right to left (products to reactants) is called backward reaction.
(iii)a reaction in which the rate of forward reaction is equal to the rate of backward reaction is called a dynamic equilibrium.
A dynamic equilibrium is therefore a balance of the rate of formation of products and reactants.
this balance continues until the reactants or products are disturbed/chamber/ altered.
the influence of different factors on a dynamic equilibrium was first investigated from 1850-1936 by the French chemist Louis Henry Le Chatellier.
His findings were called Le Chatelliers Principle which states that:
“if a stress/Change is applied to a system in dynamic equilibrium, the system readjust/shift/move/behaveso as to remove/ reduce/ counteract/ oppose the stress/Change” Le Chatelliers Principle is applied in determining the effect/influence of several factors on systems in dynamic equilibrium.
the following are the main factors that influence /alter/ affect systems in dynamic equilibrium:
(a)Concentration
(b)Pressure
(c)Temperature
(d)Catalyst
(a)Influence of concentration on dynamic equilibrium
An increase/decrease in concentration of reactants/products at equilibrium is a stress.
From Le Chatelliers principle the system redjust so as to remove/add the excessreduced concentration.
Examples of influence of concentration on dynamic equilibrium (i)CHromate(VI)/CrO42- ions in solution are yellow.
dichromate(VI)/Cr2O72- ions in solution are orange. the two solutions exist in equilibrium as in the equation:
2H+ (aq) + 2CrO42- (aq) Cr2O72- (aq) + H2O(l)
(Yellow) (Orange)
I. If an acid is/H+ (aq) is added to the equilibrium mixture a stress is created on the reactant side where there is already H+ ions.
the equilibrium shift forward to the right to remove/reduce the excess H+ ions added.
Solution mixture becomes More Cr2O72- ions formed in the solution mixture make it to be more orange in colour.
II. If a base/OH- (aq) is added to the equilibrium mixture a stress is created on the reactant side on the H+ ions. H+ ions react with OH- (aq) to form water.
the equilibrium shift backward to the left to add/replace the H+ ions that havereacted with the OH- (aq) ions .
More of the CrO42- ions formed in the solution mixture makes it to be more yellow in colour.
aa the equilibrium shift backward to the left to add/replace the 2OH- (aq) that havereacted with the H+ (aq) ions . More Cr2O72- (aq)ions formed in the solution mixture makes it to be more Orange in colour.
II. If a base /OH- (aq) is added to the equilibrium mixture a stress is created on the reactant side where there is already OH- (aq) ions.
the equilibrium shift forward to the right to remove/reduce the excess OH- (aq) ions added.
More of the Cr2O72- ions are formed in the solution mixture making it to be more orange in colour.
(i)Practical determination of the influence of alkali/acid on Cr2O72-- / CrO42- equilibrium mixture
Measure about 2 cm3 of Potassium dichromate (VI) solution into a test tube. Note that the solution mixture is orange.
Add three drops of 2M Sulphuric(VI) acid. Shake the mixture carefully.
Note that the solution mixture is remains orange.
Add about six drops of 2M sodium Hydroxide solution. Shake carefully.
Note that the solution mixture is turns yellow.
Explanation
the above observations can be explained from the fact that both the dichromate(VI)and cHromate(VI) exist in equilibrium.
dichromate(VI) ions are stable in acidic solutions while chromate(VI)ions are stable in basic solutions. An equilibrium exist thus:
OH-
H+
when an acid is added, the equilibrium shift forward to the right and the mixture become more orange as more Cr2O72- ions exist.
when a base is added, the equilibrium shift backward to the left and the mixture become more yellow as more CrO42- ions exist.
(ii)Practical determination of the influence of alkali/acid on bromine water in an equilibrium mixture
Measure 2cm3 of bromine water into a boiling tube. Note its colour. Bromine water is yellow
Add three drops of 2M Sulphuric(VI)acid. Note any colour Change
Colour becomes more yellow
Add seven drops of 2M sodium Hydroxide solution. Note any colour Change.
Solution mixture becomes colourless/Bromine water is decolourized.
Explanation
when added distilled water,an equilibrium exist between bromine liquid (Br2(aq)) and the bromide ion(Br-), Hydrobromite ion(OBr-) and hydrogen ion(H+) as in the equation:
If an acid (H+)ions is added to the equilibrium mixture, it increases the concentration of the ions on the product side which shift backwards to the left to remove the excess H+ ions on the product side making the colour of the solution mixture more yellow.
If a base/alkali OH- is added to the equilibrium mixture, it reacts with H+ ions on the product side to form water.
thisdecreases the concentration of the H+ ions on the product side which shift the equilibrium forward to the right to replace H+ ions making the solution mixture colourless/less yellow (Bromine water is decolorized)
(iii)Practical determination of the influence of alkali/acid on common acid-base indicators.
Place 2cm3 of pHenolphthalein ,methyl orange and litmus solutions each in three separate test tubes.
To each test tube add two drops of water.
Record your observations in Table 1 below.
To the same test tubes, add three drops of 2M Sulphuric(VI)acid. Record your observations in
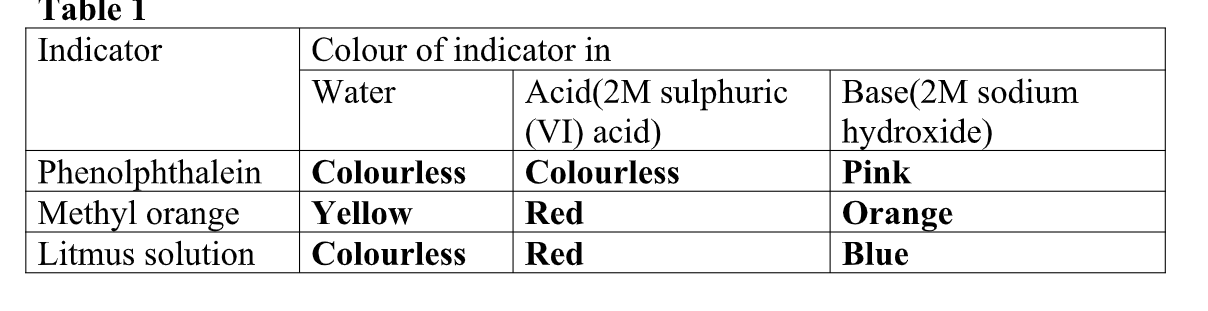
An indicator is a substance which shows whether another substance is an acid , base or neutral.
Most indicators can be regarded as very weak acids that are partially dissociated into ions.
An equilibrium exist between the undissociated molecules and the dissociated anions.
both the molecules and anions are coloured. i.e.
HIn(aq) H+ (aq) + In- (aq)
(undissociated indicator (dissociated indicator
molecule(coloured)) molecule(coloured))
when an acid H+ is added to an indicator, the H+ ions increase and equilibrium shift backward to remove excess H+ ions and therefore the colour of the undissociated (HIn) molecule shows/appears.
when a base/alkali OH- is added to the indicator, the OH- reacts with H+ ions from the dissociated indicator to form water.
(from indicator) (from alkali/base)
the equilibrium shift forward to the right to replace the H+ ion and therefore the colour of dissociated (In-) molecule shows/appears.
the following examples illustrate the above.
(i)PHenolphthalein indicator exist as:
(colourless molecule) (Pink anion)
On adding an acid ,equilibrium shift backward to the left to remove excess H+ ions and the solution mixture is therefore colourless.
when a base/alkali OH- is added to the indicator, the OH- reacts with H+ ions from the dissociated indicator to form water.
(from indicator) (from alkali/base)
the equilibrium shift forward to the right to replace the removed/reduced H+ ions. the pink colour of dissociated (PH-) molecule shows/appears.
(ii)MetHyl Orange indicator exists as:
(Red molecule) (Yellow/Orange anion)
On adding an acid ,equilibrium shift backward to the left to remove excess H+ ions and the solution mixture is therefore red.
when a base/alkali OH- is added to the indicator, the OH- reacts with H+ ions from the dissociated indicator to form water.
the equilibrium shift forward to the right to replace the removed/reduced H+ ions. the Orange colour of dissociated (Me-) molecule shows/appears.
(b)Influence of Pressure on dynamic equilibrium
Pressure affects gaseous reactants/products. Increase in pressure shift/favours the equilibrium towards the side with less volume/molecules.
Decrease in pressure shift the equilibrium towards the side with more volume/molecules.
More yield of products is obtained if High pressures produce less molecules / volume of products are formed.
If the products and reactants haveequal volume/molecules then pressure has no effect on the position of equilibrium
the following examples show the influence of pressure on dynamic equilibrium:
(i)Nitrogen(IV)oxide /Dinitrogen tetroxide mixture
Nitrogen(IV)oxide and dinitrogen tetraoxide can exist in dynamic equilibrium in a closed test tube. Nitrogen(IV)oxide is a brown gas. Dinitrogen tetraoxide is a yellow gas.
chemical equation : 2NO(g) ===== N2 O4 (g)
Gay Lussacs law 2Volume 1Volume
Avogadros law 2molecule 1molecule
2 volumes/molecules of Nitrogen(IV)oxide form 1 volumes/molecules of dinitrogen tetraoxide Increase in pressure shift the equilibrium forward to the left where there is less volume/molecules.
The equilibrium mixture become more yellow.
Decrease in pressure shift the equilibrium backward to the right where there is more volume/molecules. the equilibrium mixture become more brown.
(ii)Iodine vapour-hydrogen gas/hydrogen Iodide mixture.
Pure hydrogen gas reacts with Iodine vapour to form hydrogen Iodide gas.
chemical equation : I2(g) + H2(g) ===== 2HI (g)
Gay Lussacs law 1Volume 1Volume 2Volume
Avogadros law 1molecule 1molecule 2molecule
(1+1) 2 volumes/molecules of Iodine and hydrogen gasform 2 volumes/molecules of hydrogen Iodide gas.
Change in pressure thus has no effect on position of equilibrium.
(iii)Haber process.
Increase in pressure of the Nitrogen/hydrogen mixture favours the formation of more molecules of Ammonia gas in Haber process.
the yield of ammonia is thus favoured by High pressures
chemical equation : N2(g) + 3H2 (g) -> 2NH3 (g)
Gay Lussacs law 1Volume 3Volume 2Volume
Avogadros law 1molecule 3molecule 2molecule
(1 + 3) 4 volumes/molecules of Nitrogen and hydrogen react to form 2 volumes/molecules of ammonia.
Increase in pressure shift the equilibrium forward to the left where there is less volume/molecules.
the yield of ammonia increase.
Decrease in pressure shift the equilibrium backward to the right where there is more volume/molecules.
the yield of ammonia decrease.
(iv)Contact process.
Increase in pressure of the SulpHur(IV)oxide/Oxygen mixture favours the formation of more molecules of Sulphur(VI)oxide gas in Contact process.
the yield of Sulphur(VI)oxide gas is thus favoured by High pressures.
chemical equation : 2SO(g) + O (g) -> 2SO3 (g)
Gay Lussacs law 2Volume 1Volume 2Volume
Avogadros law 2molecule 1molecule 2molecule
(2 + 1) 3 volumes/molecules of SulpHur(IV)oxide/Oxygen mixture react to form 2 volumes/molecules of Sulphur(VI)oxide gas.
Increase in pressure shift the equilibrium forward to the left where there is less volume/molecules.
The yield of Sulphur(VI)oxide gas increase.
Decrease in pressure shift the equilibrium backward to the right where there is more volume/molecules.
the yield of Sulphur(VI)oxide gas decrease.
(v)Ostwalds process.
Increase in pressure of the Ammonia/Oxygen mixture favours the formation of more molecules of Nitrogen(II)oxide gas and water vapour in Ostwalds process.
the yield of Nitrogen(II)oxide gas and water vapour is thus favoured by low pressures.
chemical equation : 4NH3(g) + 5O (g) -> 4NO(g) + 6H2O (g)
Gay Lussacs law 4Volume 5Volume 4Volume 6Volume
Avogadros law 4molecule 5molecule 4molecule 6Molecule
(4 + 5) 9 volumes/molecules of Ammonia/Oxygen mixture react to form 10 volumes/molecules of Nitrogen(II)oxide gas and water vapour.
Increase in pressure shift the equilibrium backward to the left where there is less volume/molecules.
the yield of Nitrogen(II)oxide gas and water vapour decrease.
Decrease in pressure shift the equilibrium forward to the right where there is more volume/molecules.
the yield of Nitrogen(II)oxide gas and water vapour increase.
Note
If the water vapour is condensed on cooling, then:
chemical equation : 4NH3(g) + 5O (g) -> 4NO(g) + 6H2O (l)
Gay Lussacs law 4Volume 5Volume 4Volume 0Volume
Avogadros law 4molecule 5molecule 4molecule 0Molecule
(4 + 5) 9 volumes/molecules of Ammonia/Oxygen mixture react to form 4 volumes/molecules of Nitrogen(II)oxide gas and no vapour.
Increase in pressure shift the equilibrium forward to the right where there is less volume/molecules.
the yield of Nitrogen(II)oxide gas increase.
Decrease in pressure shift the equilibrium backward to the left where there is more volume/molecules. the yield of Nitrogen(II)oxide gas decrease.
(c)Influence of Temperature on dynamic equilibrium
A decrease in temperature favours the reaction that liberate/generate more Heat thus exothermic reaction(-ΔH).
An increase in temperature favours the reaction that do not liberate /generate more Heat thus endothermic reaction(+ΔH).
Endothermic reaction are thus favoured by High temperature/heating
Exothermic reaction are favoured by low temperature/cooling.
If a reaction/equilibrium mixture is neither exothermic or endothermic, then a Change in temperature/cooling/heating has no effect on the equilibrium position.
(i)Nitrogen(IV)oxide /Dinitrogen tetroxide mixture
Nitrogen(IV)oxide and dinitrogen tetraoxide can exist in dynamic equilibrium in a closed test tube. Nitrogen(IV)oxide is a brown gas. Dinitrogen tetraoxide is a yellow gas.
chemical equation : 2NO(g) ===== N2 O4 (g)
On heating /increasing temperature, the mixture becomes more brown. On cooling the mixture become more yellow.
thisshow that
(i)the forward reaction to the right is exothermic(-ΔH).
On heating an exothermic process the equilibrium shifts to the side that generate /liberate less Heat.
(ii)the backward reaction to the right is endothermic(+ΔH).
On cooling an endothermic process the equilibrium shifts to the side that do not generate /liberate Heat.
(c)Influence of Catalyst on dynamic equilibrium
A catalyst has no effect on the position of equilibrium. It only speeds up the rate of attainment. e.g.
Esterification of alkanols and alkanoic acids naturally take place in fruits.In the laboratory concentrated Sulphuric(VI)acid catalyse the reaction.
The equilibrium mixture forms the ester faster but the yield does not increase.
Industrial processes are commercial profit oriented. All industrial processes take place in closed systems and thus in dynamic equilibrium.
For manufacturers, obtaining the Highest yield at minimum cost and shortest time is paramount.
the conditions required to obtain the Highest yield of products within the shortest time at minimum cost are called optimum conditions
Optimum condition thus require understanding the effect of various factors on:
(i)rate of reaction(chemical kinetics)
(ii)dynamic equilibrium(chemical cybernetics)
1.Optimum condition in Haber process chemical equation
Equilibrium/Reaction rate considerations
(i)Removing ammonia gas once formed shift the equilibrium forward to the right to replace the ammonia. More/higher yield of ammonia is attained.
(ii)Increase in pressure shift the equilibrium forward to the right where there is less volume/molecules .
More/higher yield of ammonia is attained.
Very High pressures raises the cost of production because they are expensive to produce and maintain.
An optimum pressure of about 500 atmospheres is normally used.
(iii)Increase in temperature shift the equilibrium backward to the left because the reaction is exothermic(ΔH = -92kJ) .
Ammonia formed decomposes back to Nitrogen and hydrogen to remove excess Heat therefore a less yield of ammonia is attained.
Very low temperature decrease the collision frequency of Nitrogen and hydrogen and thus the rate of reaction too slow and uneconomical.
An optimum temperature of about 450oC is normally used.
(iv)Iron and platinum can be used as catalyst. Platinum is a better catalyst but more expensive and easily poisoned by impurities than Iron.
Iron is promoted /impregnated with AluminiumOxide(Al2O3) to increase its surface area/area of contact with reactants and thus efficiency.
The catalyst does not increase the yield of ammonia but it speed up its rate of formation.
2.Optimum condition in Contact process
chemical equation
Equilibrium/Reaction rate considerations
(i)Removing Sulphur(VI)oxide gas once formed shift the equilibrium forward to the right to replace the sulpHur(VI)oxide. More/higher yield of sulpHur(VI) oxide is attained.
(ii)Increase in pressure shift the equilibrium forward to the right where there is less volume/molecules .
More/higher yield of sulpHur(VI)oxide is attained.
Very High pressures raises the cost of production because they are expensive to produce and maintain.
An optimum pressure of about 1-2 atmospheres is normally used to attain about 96% yield of SO3.
(iii)Increase in temperature shift the equilibrium backward to the left because the reaction is exothermic(ΔH = -197kJ) .
SulpHur(VI)oxide formed decomposes back to SulpHur(IV)oxide and Oxygen to remove excess Heat therefore a less yield of SulpHur(VI)oxide is attained.
Very low temperature decrease the collision frequency of SulpHur(IV)oxide and Oxygen and thus the rate of reaction too slow and uneconomical.
An optimum temperature of about 450oC is normally used.
(iv)Vanadium(V)Oxide and platinum can be used as catalyst. Platinum is a better catalyst and less easily poisoned by impurities but more expensive.
Vanadium(V)Oxide is very cHeap even if it is easily poisoned by impurities. the catalyst does not increase the yield of SulpHur (VI)Oxide but it speed up its rate of formation.
3.Optimum condition in Ostwalds process
chemical equation
Equilibrium/Reaction rate considerations
(i)Removing Nitrogen(II)oxide gas once formed shift the equilibrium forward to the right to replace the Nitrogen(II)oxide.
More/higher yield of Nitrogen(II) oxide is attained.
(ii)Increase in pressure shift the equilibrium backward to the left where there is less volume/molecules .
Less/lower yield of Nitrogen(II)oxide is attained.
Very low pressures increases the distance between reacting NH3and O molecules.
An optimum pressure of about 9 atmospheres is normally used.
(iii)Increase in temperature shift the equilibrium backward to the left because the reaction is exothermic(ΔH = -950kJ) .
Nitrogen(II)oxide and water vapour formed decomposes back to Ammonia and Oxygen to remove excess Heat therefore a less yield of Nitrogen(II)oxide is attained.
Very low temperature decrease the collision frequency of Ammonia and Oxygen and thus the rate of reaction too slow and uneconomical.
An optimum temperature of about 900oc is normally used.
(iv)Platinum can be used as catalyst. Platinum is very expensive.It is:
- Promoted with Rhodium to increase the surface area/area of contact.
-added/coated on the surface of asbestos to form platinized –asbestos to reduce the amount/quantity used.
the catalyst does not increase the yield of Nitrogen (II)Oxide but it speed up its rate of formation.
C.sample Revision Questions
1.State two distinctive features of a dynamic equilibrium.
(i)the rate of forward reaction is equal to the rate of forward reaction (ii)at equilibrium the concentrations of reactants and products do not Change.
2. Explain the effect of increase in pressure on the following:
(i) N2(g) + O(g) ===== 2NO(g)
Gay Lussacs law 1Volume 1Volume 2 Volume
Avogadros law 1 molecule 1 molecule 2 molecule
2 volume on reactant side produce 2 volume on product side.
Increase in pressure thus haveno effect on position of equilibrium.
(ii) 2H2(g) + CO(g) ===== CHOH (g)
Gay Lussacs law 2Volume 1Volume 1 Volume
Avogadros law 2 molecule 1 molecule 1 molecule
3 volume on reactant side produce 1 volume on product side.
Increase in pressure shift the equilibrium forward to the left. More yield of CHOH is formed.
4. Explain the effect of increasing temperature on the following:
2SO(g) + O (g) ===== 2SO3 (g) ΔH = -189kJ
Forward reaction is exothermic. Increase in temperature shift the equilibrium backward to reduce the excess Heat.
5.120g of brass an alloy of copper and Zinc was put it a flask containing dilute Hydrochloric acid.
the flask was placed on an electric balance.
the readings on the balance were recorded as in the table below
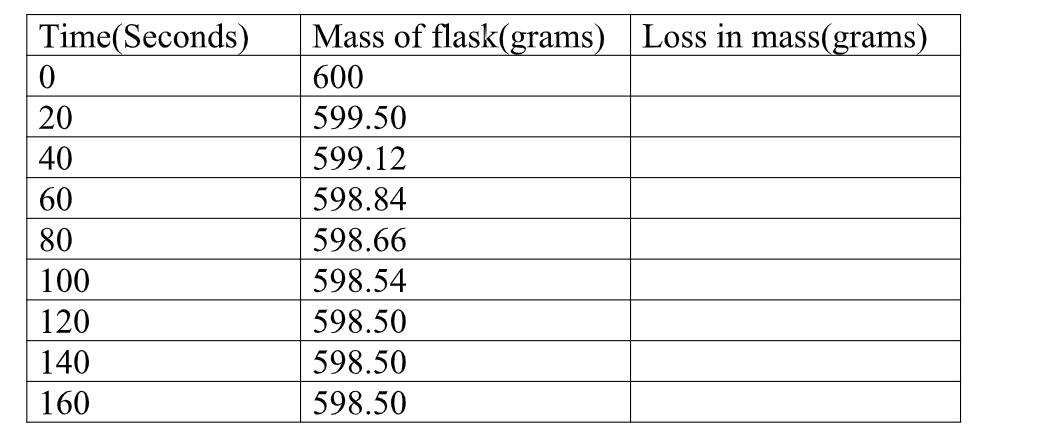
(b)what does the “600” gram reading on the balance represent the initial mass of brass and the acid before any reaction take place.
(c)Plot a graph of Time (x-axes) against loss in mass.
(d)Explain the shape of your graph
the reaction produce hydrogen gas as one of the products that escape to the atmosphere. thisdecreases the mass of flask.
After 120 seconds,the react is complete. No more hydrogen is evolved.The mass of flask remain constant.
(d)At what time was the loss in mass equal to:
(i)1.20g
Reading from a correctly plotted graph =
(ii)1.30g
Reading from a correctly plotted graph =
(iii)1.40g
Reading from a correctly plotted graph =
(e)what was the loss in mass at:
(i)50oC
Reading from a correctly plotted graph =
(ii) 70oc
Reading from a correctly plotted graph =
(iii) 90oc g
Reading from a correctly plotted graph =
ElectrocHemistry
ElectrocHemistry can be defined as the study of the effects of electricity on a substance/ compound and How chemical reactions produce electricity. ElectrocHemistry therefore deals mainly with:
i) Reduction and oxidation
ii) electrochemical (voltaic) cell
iii) Electrolysis (electrolytic) cell
(i)reduction and Oxidation (Redox)
1. In teams of oxygen transfer:
i) Reduction is removal of oxygen.
ii) Oxidation is addition of oxygen.
iii) Redox is simultaneous addition and removal of oxygen.
iv) Reducing agent is the species that undergoes oxidation, therefore gains oxygen.
v) Oxidizing agent is the species that undergoes reduction, therefore looses/donates oxygen.
e.g. when hydrogen is passed throughheated copper (II) oxide, it is oxidised to copper metal as in the equation below:
2. In terms of hydrogen transfer:
i) Oxidation is the removal of hydrogen.
ii) Reduction is the addition of hydrogen.
iii) Redox is simultaneous addition and removal of hydrogen.
iv) Reducing agent is the species that undergoes oxidation, therefore looses/ donates hydrogen.
v) Oxidizing agent is the species that undergoes reduction, therefore gains hydrogen.
e.g. when hydrogen sulphide gas is bubbled into a gas jar containing Chlorine gas it is oxidized (loose the hydrogen) to sulpHur (yellow solid). the Chlorine is reduced (gain hydrogen) to hydrogen Chlorine gas.
3. In terms of electron transfer:
i) Oxidation is donation/ loss/ removal of electrons.
ii) Reduction is gain/ accept/ addition of electrons.
iii) Redox is simultaneous gain/ accept/ addition and donation/ loss/ removal of electrons.
iv) Reducing agent is the species that undergoes oxidation, therefore looses/ donates electrons.
v) Oxidizing agent is the species that undergoes reduction, therefore gains/ accepts electrons.
Example
a) Displacement of metals from their solutions:
Place 5cm3 eachof Iron (II) Sulphate (VI) solution into three different test tubes.
Add about 1g of copper tunings / powder into one test tube then zinc and magnesium powders separately into the other test tubes.
Shake thoroughly for 2 minutes each. Record any colour changes in the table below.
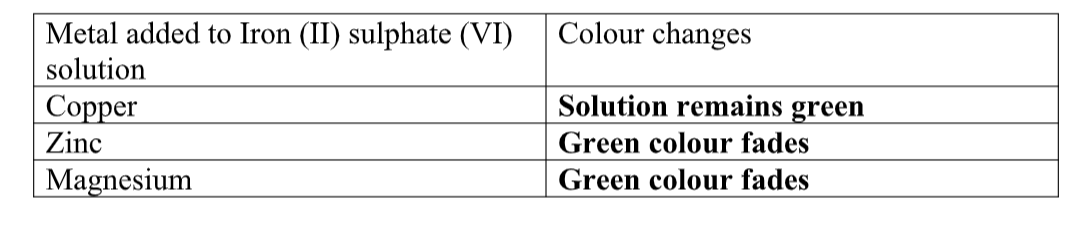
-when a more reactive metal is added to a solution of less reactive metal, it displaces it from its solution.
-when a less reactive metal is added to a solution of a more reactive metal, it does not displace it from its solution.
-Copper is less reactive than iron therefore cannot displace iron its solution.
-Zinc is more reactive than iron therefore can displace iron from its solution.
-Magnesium is more reactive than iron therefore can displace iron from its solution.
In terms of electron transfer:
- the more reactive metal undergoes oxidation (reducing agent) by donating/loosing electrons to form ions
- The less reactive metal undergoes reduction (oxidizing agent) by its ions in solution gaining /accepting/acquiring the electrons to form the metal.
-displacement of metals involves therefore electron transfer from a more reactive metal to ions of another less reactive metal.
Examples
1. Zn(s) -> Zn2+(aq) + 2e (oxidation/donation of electrons)
Fe2+(aq) + 2e -> Fe(s) (reduction/gain of electrons)
Fe2+(aq) + Zn(s) -> Zn2+(aq) + Fe(s) (redox/both donation and gain of electrons)
2. Mg(s) -> Mg2+(aq) + 2e (oxidation/donation of electrons)
Fe2+(aq) + 2e -> Fe(s) (reduction/gain of electrons)
Fe2+(aq) + Mg(s) -> Mg2+(aq) + Fe(s) (redox/both donation and gain of electrons)
3. Zn(s) -> Zn2+(aq) + 2e (oxidation/donation of electrons)
Cu2+(aq) + 2e -> Cu(s) (reduction/gain of electrons)
Cu2+(aq) + Zn(s) -> Zn2+(aq) + Cu(s) (redox/both donation and gain of electrons)
4. Fe(s) -> Fe2+(aq) + 2e (oxidation/donation of electrons)
2Ag+(aq) + 2e -> 2Ag(s) (reduction/gain of electrons)
2Ag+(aq) + Fe(s) -> Fe2+(aq) + 2Ag(s) (redox/both donation and gain of electrons)
5. Zn(s) -> Zn2+(aq) + 2e (oxidation/donation of electrons)
Cl2(g) + 2e -> 2Cl-(aq) (reduction/gain of electrons)
Cl2(g) + Zn(s) -> Zn2+(aq) + 2Cl-(aq) (redox/both donation and gain of electrons)
6. 2Mg(s) -> 2Mg2+(aq) + 4e (oxidation/donation of electrons)
O2(g) + 4e -> 2O2-(aq) (reduction/gain of electrons)
O2(g) + 2Mg(s) -> 2Mg2+(aq) + 2O2-(aq) (redox/both donation and gain of electrons)
Note
(i)the number of electrons donated/lost MUST be equal to the number of electrons gained/acquired.
(i)During displacement reaction, the colour of ions /salts fades but does not if displacement does not take place. e.g
a)Green colour of Fe2+(aq) fades if Fe2+(aq) ions are displaced from their solution.
Green colour of Fe2+(aq) appear if Fe/iron displaces another salt/ions from their solution.
b)Blue colour of Cu2+(aq) fades if Cu2+(aq) ions are displaced from their solution and brown copper deposits appear.
Blue colour of Cu2+(aq) appear if Cu/copper displaces another salt/ions from their solution.
c)Brown colour of Fe3+(aq) fades if Fe3+(aq) ions are displaced from their solution.
Brown colour of Fe3+(aq) appear if Fe/iron displaces another salt/ions from their solution to form Fe3+(aq).
(iii)Displacement reactions also produce energy/Heat.
the closer/nearer the metals in the reactivity/electrochemical series the less energy/Heat of displacement.
(iv)the higher the metal in the reactivity series therefore the easier to loose/donate electrons and thus the stronger the reducing agent.
4. (a)In terms of oxidation number:
i) Oxidation is increase in oxidation numbers.
ii) Reduction is decrease in oxidation numbers.
iii) Redox is simultaneous increase in oxidation numbers of one species/substance and a decrease in oxidation numbers of another species/substance.
iv) Reducing agent is the species that undergoes oxidation, therefore increases its oxidation number.
v) Oxidizing agent is the species that undergoes reduction, therefore increases its oxidation number.
(b)the idea/concept of oxidation numbers uses/applies the following simple guideline rules:
Guidelines /rules applied in assigning oxidation number
1.Oxidation number of combined Oxygen is always -2 except in peroxides (Na2O2/H2O2) where its Oxidation number is -1
2.Oxidation number of combined hydrogen is always +1except in Hydrides (NaH/KH) where its Oxidation number is -1
3.All atoms and molecules of elements haveoxidation number 0 (zero)
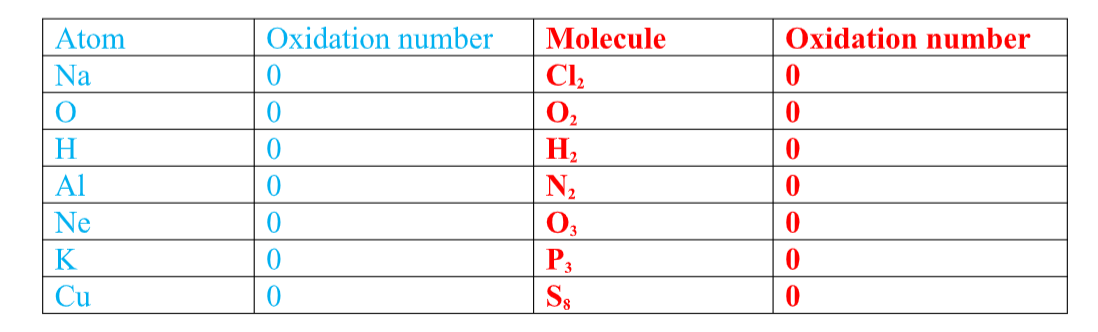
Metal/non-metal ion Valency Oxidation state Oxidation number
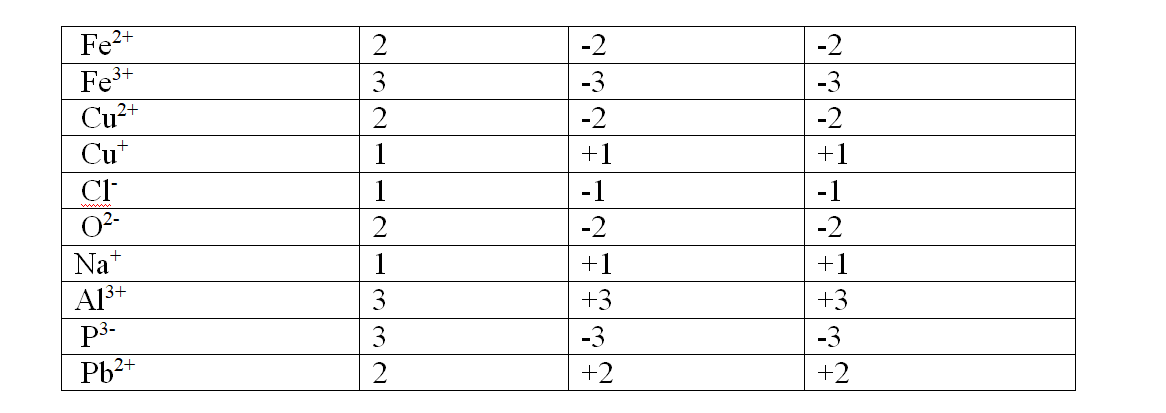
Using thisrule ,an unknown oxidation number of an atom in a compound can be determined as below:
a) CuSO4 has-
-one atom of Cu with oxidation number +2( refer to Rule 4)
-one atom of S with oxidation number +6 ( refer to Rule 4)
-six atoms of O eachwith oxidation number -2( refer to Rule 4)
Sum of oxidation numbers of atoms in CuSO4 = (+2 + +6 + (-2 x 6)) = 0
b) H2SO4 has-
- Two atom of H eachwith oxidation number +1( refer to Rule 2)
-one atom of S with oxidation number +6 ( refer to Rule 4)
-four atoms of O eachwith oxidation number -2( refer to Rule 4)
Sum of oxidation numbers of atoms in H2SO4 = (+2 + +6 + (-2 x 4)) = 0
c) KMnO4 has-
-one atom of K with oxidation number +1( refer to Rule 4)
-one atom of Mn with oxidation number +7 ( refer to Rule 4)
-four atoms of O eachwith oxidation number -2( refer to Rule 4)
Sum of oxidation numbers of atoms in KMnO4= (+1 + +7 + (-2 x 4)) = 0
Determine the oxidation number of:
I.Nitrogen in;
-NO => x + -2 = 0 thus x = 0 – (-2) = + 2
the chemical name of this compound is thus Nitrogen(II)oxide
-NO2 => x + (-2 x2)= 0 thus x = 0 – (-4) = + 4
the chemical name of this compound is thus Nitrogen(IV)oxide
-N2O => 2x + -2 = 0 thus 2x = 0 – (-2) = +2/2= +1
the chemical name of this compound is thus Nitrogen(I)oxide
II. SulpHur in;
-SO2 => x + (-2 x2)= 0 thus x = 0 – (-4) = + 4
the chemical name of this compound is thus SulpHur(IV)oxide
-SO3 => x + (-2 x3)= 0 thus x = 0 – (-6) = + 6
the chemical name of this compound is thus SulpHur(VI)oxide
-H2SO4 = ((+1 x 2) + x + (-2 x 4)) thus x= 0-( +2 +-8) =+6
the chemical name of this compound is thus Sulphuric(VI)acid
-H2SO3 = ((+1 x 2) + x + (-2 x 3)) thus x= 0-( +2 +-6) =+4
the chemical name of this compound is thus Sulphuric(IV)acid
III. Carbon in;
-CO2 => x + (-2 x2)= 0 thus x = 0 – (-4) = + 4 the chemical name of this compound is thus carbon(IV)oxide
-CO => x + -2 = 0 thus x = 0 – -2 = + 2
the chemical name of this compound is thus carbon(II)oxide
-H2CO3 = ((+1 x 2) + x + (-2 x 3)) thus x= 0-( +2 +-6) =+4
the chemical name of this compound is thus Carbonic(IV)acid IV.Manganese in;
-MnO2 => x + (-2 x2)= 0 thus x = 0 – (-4) = + 4
the chemical name of this compound is thus Manganese(IV)oxide
-KMnO4 = ((+1 + x + (-2 x 4)) thus x= 0-( +1 +-8) =+7
the chemical name of this compound is thus Potassium manganate(VII) V.Chromium in;
- Cr2O3 => 2x + (-2 x 3)= 0 thus 2x = 0 – (-6) = +6 / 2= +3
the chemical name of this compound is thus Chromium(III)oxide -K2Cr2O7 => (+1 x 2) + 2x + (-2 x7)= 0
thus 2x = 0 – +2 +-14 = +12 / 2= +6
the chemical name of this compound is thus Potassium dichromate(VI) -K2CrO4 => (+1 x 2) + x + (-2 x4)= 0
thus 2x = 0 – +2 +-8 = +12 / 2= +6
the chemical name of this compound is thus Potassium cHromate(VI)
6.The sum of the oxidation numbers of atoms of elements making a Charged radical/complex ion is equal to its Charge.
Using thisrule ,the oxidation number of unknown atom of an element in a Charged radical/complex ion can be determined as in the examples below;
a) SO42- has-
-one atom of S with oxidation number +6( refer to Rule 4)
-four atoms of O eachwith oxidation number -2( refer to Rule 1)
Sum of oxidation numbers of atoms in SO42- = ( +6 + (-2 x 4)) = -2
the chemical name of thisradical is thus Sulphate(VI) ion
b) NO3- has-
-one atom of N with oxidation number +4( refer to Rule 4)
- Three atoms of O eachwith oxidation number -2( refer to Rule 1)
Sum of oxidation numbers of atoms in NO3- = ( +4 + (-2 x 3)) = -1
the chemical name of thisradical is thus nitrate(IV) ion.
Determine the oxidation number of:
I.Nitrogen in;
-NO2- => x + (-2 x2)= -1 thus x = -1 – (-4) = + 3
the chemical name of this compound/ion/radical is thus Nitrate(III)ion II. SulpHur in;
-SO32- => x + (-2 x3)= -2 thus x = -2 – (-6) = + 4
the chemical name of this compound/ion/radical is thus Sulphate(IV)ion III. Carbon in;
-CO32- = x + (-2 x 3) = -2 thus x = -2 – (-6) = + 4
the chemical name of this compound/ion/radical is thus Carbonate(IV)ion IV.Manganese in;
-MnO4 - = x + (-2 x 4)= -1 thus x= -1-(-2 +-8) =+7
the chemical name of this compound/ion/radical is thus manganate(VII) ion V.Chromium in
-Cr2O72- => 2x + (-2 x7)= -2
thus 2x = -2 – +2 +-14 = +12 / 2= +6
the chemical name of this compound/ion//radical is thus dichromate(VI) ion -CrO42- => x + (-2 x4)= -2
thus x = -2 + (-2 x 4) = +6
the chemical name of this compound/ion//radical is thus cHromate(VI) ion
(c)Using the concept/idea of oxidation numbers as increase and decrease in oxidation numbers , the oxidizing and reducing species/agents can be determined as in the following examples;
(i) Cu2+ (aq) + Zn(s) -> Zn2+ (aq) + Cu(s) Oxidation numbers -> +2 0 +2 0 Oxidizing species/agents =>Cu2+;its oxidation number decrease from+2 to 0 in Cu(s)
Reducing species/agents => Zn2+;its oxidation number increase from 0 to +2 in Zn(s)
(ii) 2Br- (aq) + Cl2(g) -> 2Cl-- (aq) + Br2 (l)
Oxidation numbers -> -1 0 -1
0 Oxidizing agent =>Cl2(g) ;its oxidation number decrease from 0 to-1 in 2Cl-- (aq)
Reducing agents => Zn2+;its oxidation number increase from -1 to 0 in Zn(s)
(iii) Br2 (l) + Zn(s) -> Zn2+ (aq) + 2Br-(aq)
Oxidation numbers -> 0 0 +2 -1
Oxidizing agent => Br2 (l) ;its oxidation number decrease from 0 to-1 in 2Br-(aq)
Reducing agents => Zn(s) ;its oxidation number increase from 0 to +2 in Zn2+
(iv) 2HCl (aq) + Mg(s) -> MgCl2 (aq) + H2 (g)
Oxidation numbers -> 2 (+1 -1) 0 +2 2(-1) 0
Oxidizing agent => H+ in HCl;its oxidation number decrease from +1to 0 in H2 (g)
Reducing agents => Mg(s) ;its oxidation number increase from 0 to +2 in Mg2+
(v) 2H22O (l) + 2Na(s) -> 2NaOH (aq) + H2 (g)
Oxidation numbers -> +1 -2 0 +1 -2 +1 0
Oxidizing agent => H+ in H2O;its oxidation number decrease from +1to 0 in H2 (g)
Reducing agents => Na(s) ;its oxidation number increase from 0 to +1 in Na+
(vi) 5Fe2+ (aq) + 8H+ (aq) + MnO4- -> 5Fe3+(aq) + Mn2+ (aq) + 4H2O (l) +2 +1 +7 -2 +3 +2 +1 -2
Oxidizing agent => Mn in MnO4- ;its oxidation number decrease from +7to+2 in Mn2+
Reducing agents => Fe2+ ;its oxidation number increase from +2 to +3 in Fe3+
(vii) 6Fe2+ (aq) + 14H+ (aq) + Cr2O72-(aq) -> 6Fe3+(aq) + Cr3+ (aq) + 7H2O (l) +2 +1 +6 -2 +3 +3 +1 -2
Oxidizing agent:
Cr in Cr2O72- ;its oxidation number decrease from +6 to+3 in Cr3+
Reducing agents => Fe2+ ;its oxidation number increase from +2 to +3 in Fe3+
(viii) 2Fe2+ (aq) + 2H+ (aq) + H2O2(aq) -> 2Fe3+(aq) + 2H22O (l)
+2 +1 +1 -1 +3 +1 -2
Oxidizing agent:
O in H2O2;its oxidation number decrease from -1 to -2 in H2O
Reducing agents => Fe2+ ;its oxidation number increase from +2 to +3 in Fe3+
(ix) Cr2O72-(aq) + 6H+ (aq) + 5H2O2(aq) -> 2Cr3+ (aq) + 2H22O (l) + 5O2(g) +6 -2 +1 +1 -1 +3 +1 -2 0
Oxidizing agents:
O in H2O2;its oxidation number decrease from -1 to -2 in H2O
Cr in Cr2O72- its oxidation number decrease from +6 to +3 in Cr3+
Reducing agents
O in H2O2;its oxidation number increase from -1 to O in O2(g)
O in Cr2O72- its oxidation number increase from -2 to O in O2(g)
(x) 2MnO4-(aq) + 6H+ (aq) + 5H2O2(aq) -> 2Mn2+ (aq) + 8H2O (l) + 5O2(g) +7 -2 +1 +1 -1 +2 +1 -2 0
Oxidizing agents:
O in H2O2;its oxidation number decrease from -1 to -2 in H2O
Mn in MnO4- its oxidation number decrease from +7 to +2 in Mn2+
Reducing agents
O in H2O2;its oxidation number increase from -1 to O in O2(g)
O in MnO4- its oxidation number increase from -2 to O in O2(g)
(ii)electrochemical (VOLTAIC) CELL
1. when a metal rod/plate is put in a solution of its own salt, some of the metal ionizes and dissolve into the solution i.e.
2.The metal rod becomes therefore negatively Charged wHile its own solution positively Charged.
As the positive Charges of the solution increase, some of them recombine with the electrons to form back the metal atoms
the difference can be measured by connecting two half cells to form an electrochemical/voltaic cell as in the below procedure:
To set up an electrochemical /voltaic cell
To compare the relative tendency of metals to ionize
Place 50cm3 of 1M Zinc(II) Sulphate(VI) in 100cm3 beaker. Put a clean zinc rod/plate into the solution. Place 50cm3 of 1M Copper(II) Sulphate(VI) in another 100cm3 beaker.
Put a clean copper rod/plate of equal area (lengthx width) with Zinc into the solution.
Connect/join the two metals(to a voltmeter) using connecting wires.
Dip a folded filter paper into a solution of Potassium nitrate(V) or sodium(I) chloride(I) until it soaks.
Use the folded soaked filter paper to connect/join the two solutions in the two beakers.
The whole set up sHould be as below
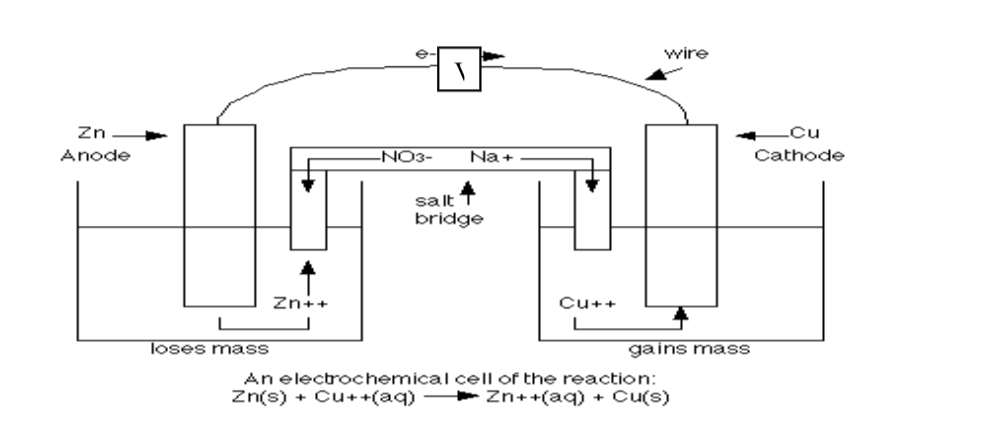
(i)Zinc half cell with Magnesium rod/plate/ribbon dipped in 50cm3 of IM magnesium (II) Sulphate(VI) solution
(ii)Zinc half cell with Silver rod/plate/coin dipped in 50cm3 of IM silver(I) nitrate(V) solution
(iii)Copper half cell with Iron rod/plate/spoon dipped in 50cm3 of IM Iron (II) Sulphate(VI) solution
Record the observations in the table below
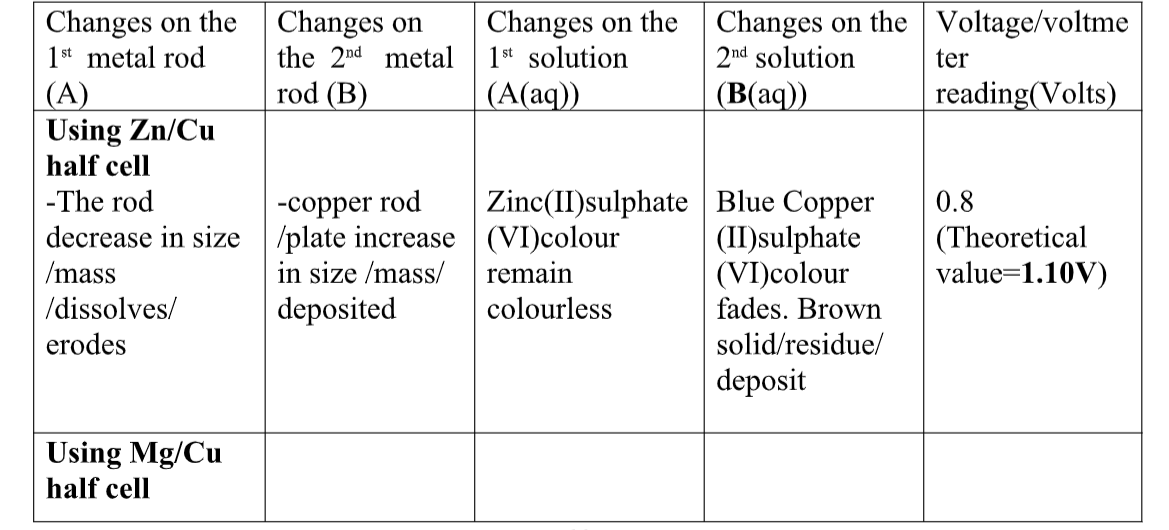
(i)in the Zn/Cu half-cell the;
-Zinc rod/plate ionizes /dissolves faster than the copper rod/plate to form Zn2+ Ionic equation Zn(s) -> Zn2+(aq) + 2e -blue copper ions in the Copper (II)Sulphate solution gains the donated electrons to form brown copper metal/atoms
Ionic equation Cu2+(aq) + 2e -> Cu(s) this reaction shows /imply the Zinc rod has a higher tendency to ionize than copper.
The Zinc rod has a higher net accumulation of electrons and is more negative compared to the copper rod which has lower accumulation of electrons.
the copper rod is therefore relatively more positive with respect to Zinc rod.
when the two half cells are connected , electrons therefore flow from the negative Zinc rod throughthe external wire to be gained by copper ions.
thismeans a net accumulation/increase of Zn2+ positive ions on the negative half cell and a net decrease in Cu2+ positive ions on the positive half cell.
the purpose of the salt bridge therefore is:
(i)complete the circuit
(ii)maintain balance of Charges /ions on both half cells.
For the negative half cell the NO3- /Cl- from salt bridge decrease/neutralise the increased positive(Zn2+) ion.
For the positive half cell the Na+ / K+ from salt bridge increase the decreased positive(Cu2+) ion.
the voltmeter should theoretically register/read a 1.10Volts as a measure of the electromotive force (e.m.f) of the cell .
Practically the voltage reading is lowered because the connecting wires have some resistance to be overcomed.
A combination of two half cells that can generate an electric current from a redox reaction is called a voltaic/electrochemical cell.
By convention a voltaic/electrochemical cell is represented;
(metal rod of M)(solution ofM)(solution ofN)(metal rod ofN) Note;
a)(i) Metal M must be the one higher in the reactivity series.
(ii) It forms the negative terminal of the cell.
(iii) It must diagrammatically be drawn first on the left Hand side when illustrating the voltaic/electrochemical cell.
b)(i)Metal N must be the one lower in the reactivity series.
(ii)It forms the positive terminal of the cell.
(iii)It must diagrammatically be drawn second/after/ right Hand side when illustrating the voltaic/electrochemical cell.
Illustration of the voltaic/electrochemical cell.
(i)Zn/Cu cell
1. Zinc rod ionizes /dissolves to form Zn2+ ions at the negative terminal
2. Copper ions in solution gain the donated electrons to form copper atoms/metal
3.Overall redox equation
4.cell representation.
5.cell diagram
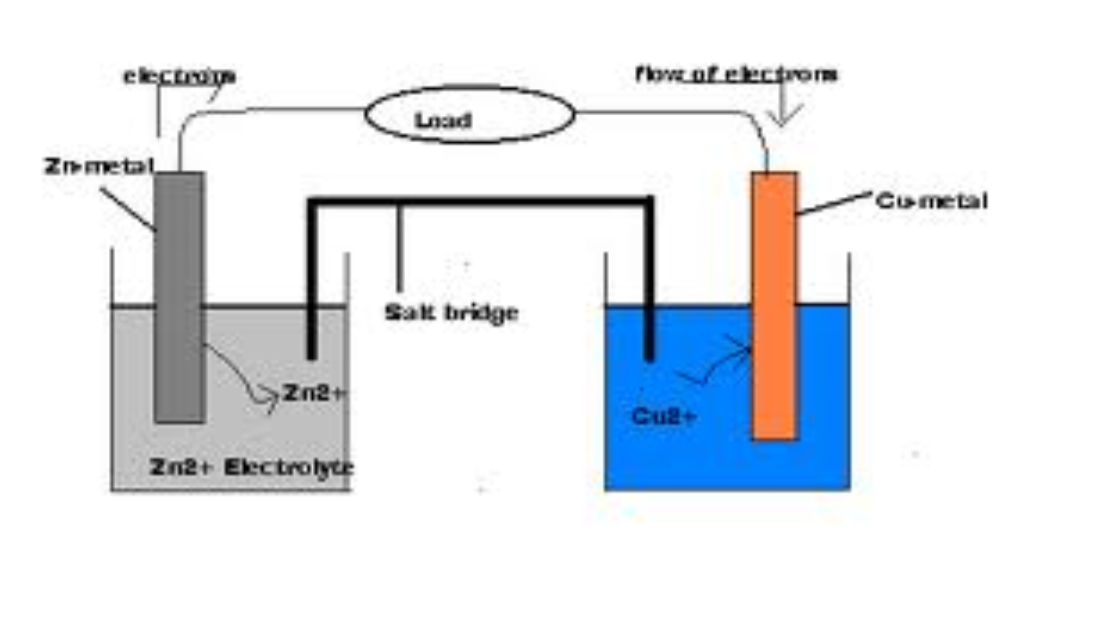
1. Magnesium rod ionizes /dissolves to form Mg2+ ions at the negative terminal
2. Copper ions in solution gain the donated electrons to form copper atoms/metal
3.Overall redox equation
4.cell representation.
5.cell diagram.
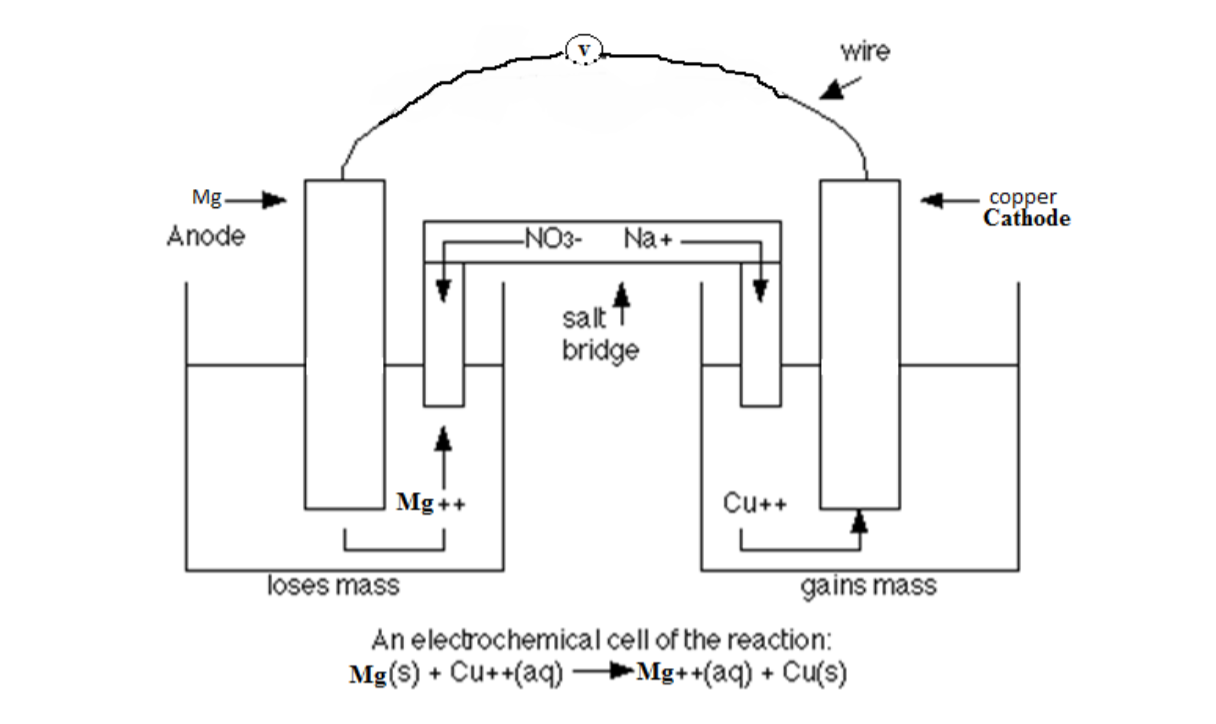
1. Magnesium rod ionizes /dissolves to form Mg2+ ions at the negative terminal
4.cell representation.
5.cell diagram.
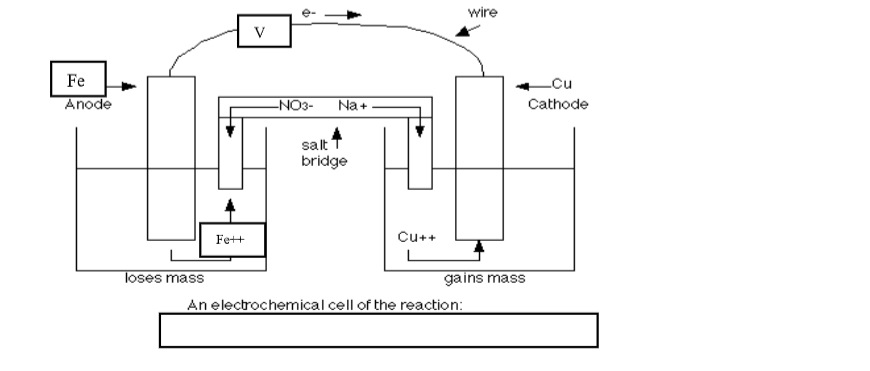
1. Copper rod ionizes /dissolves to form Cu2+ ions at the negative terminal
2. Silver ions in solution gain the donated electrons to form silver atoms/metal
4.cell representation.
5.cell diagram.
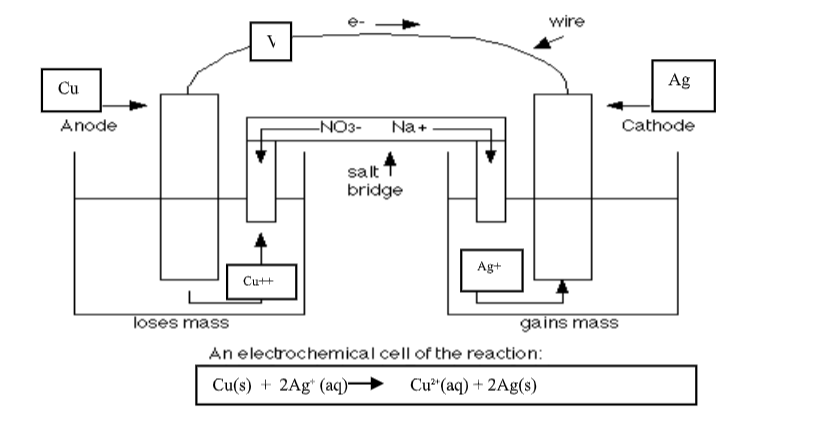
the standard electrode potential (Eᶿ) is obtained if the hydrogen half cell is used as reference.
The standard electrode potential (Eᶿ) consist of inert platinum electrode immersed/dipped in 1M solution of (Sulphuric(VI) acid) H+ ions. hydrogen gas is bubbled on the platinum electrodes at:
(i)a temperature of 25oc
(ii)atmospHeric pressure of 101300Pa/101300Nm-2/1atm/760mmHg/76cmHg (iii)a concentration of 1M(1moledm-3) of Sulphuric(VI) acid/ H+ ions and 1M(1moledm-3) of the other half cell.
hydrogen is adsorbed onto the surface of the platinum. An equilibrium/balance exist between the adsorbed layer of molecular hydrogen and H+ ions in solution to form a half cell.
½ H2 (g) ==== H+ (aq) + e
the half cell representation is:
Pt,½ H2 (g) / H+ (aq), 1M
the standard electrode potential (Eᶿ) is thus defined as the potential difference for a cell comprising of a particular element in contact with1M solution of its own ions and the standard hydrogen electrode.
If the other electrode has a higher/greater tendency to lose electrons than the hydrogen electrode, the electrode is therefore negative with respect to hydrogen electrode and its electrode potential has negative (Eᶿ) values.
If the other electrode has a lower/lesser tendency to lose electrons than the hydrogen electrode, the electrode is therefore positive with respect to hydrogen electrode and its electrode potential has positive (Eᶿ) values.
Table showing the standard electrode potential (Eᶿ) of some reactions Reactio
n (Eᶿ) values in volts
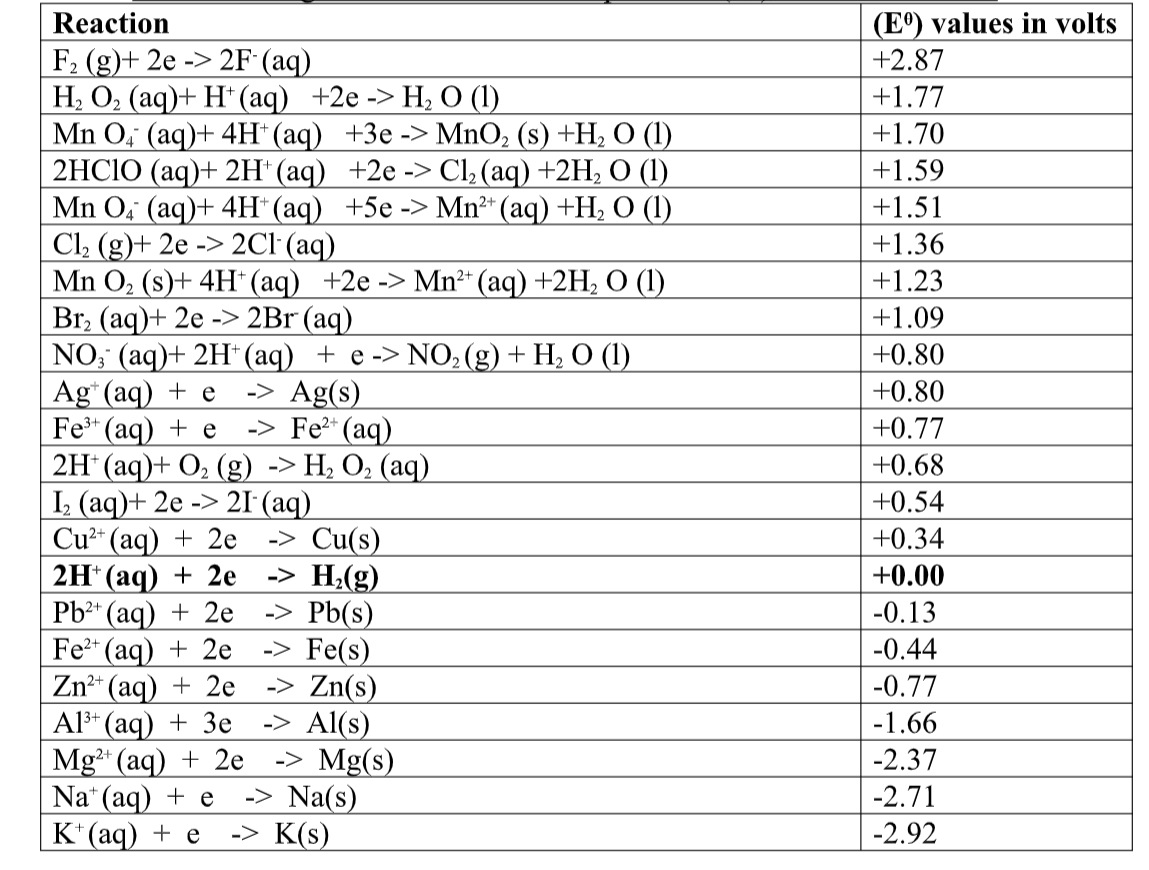
(i)Eᶿ values generally show the possibility/feasibility of a reduction process/oxidizing strength.
(ii)the element/species in the half cell with the Highest negative Eᶿ value easily gain / acquire electrons.
It is thus the strongest oxidizing agent and its reduction process is highly possible/feasible. the element/species in the half cell with the lowest positive Eᶿ value easily donate / lose electrons.
It is thus the strongest reducing agent and its reduction process is the least possible/feasible.
(iii)the overall redox reaction is possible/feasible is it has a positive (+) Eᶿ. If the overall redox reaction is not possible/ not feasible/ forced, it has a negative (-) Eᶿ
Calculation examples on Eᶿ
Calculate the Eᶿ value of a cell made of:
a)Zn and Cu
From the table above:
Overall Eᶿ = Eᶿ higher- Eᶿ lower / Eᶿ RHS - Eᶿ LHS/ Eᶿoxidized- Eᶿ reduced Substituting:
Overall Eᶿ = +0.34 – (- 0.77) = +1.10V
Overall redox equation:
Overall conventional cell representation:
Overall conventional cell diagram:
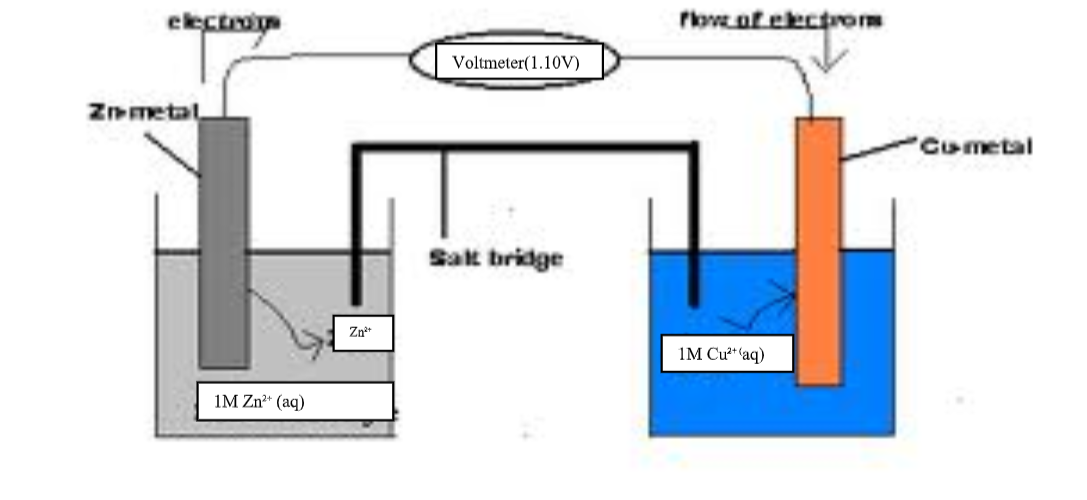
b)Mg and Cu
From the table above:
Overall Eᶿ = Eᶿ higher- Eᶿ lower / Eᶿ RHS - Eᶿ LHS/ Eᶿ oxidized- Eᶿ reduced
Substituting:
Overall Eᶿ = +0.34 – (- 2.37) = +2.71V
Overall redox equation:
Overall conventional cell representation:
Mg(s) / Mg2+ (aq) 1M, // 1M,Cu2+ (aq) / Cu(s) Eᶿ = +2.71V
c)Ag and Pb
From the table above:
Overall Eᶿ = Eᶿ higher- Eᶿ lower / Eᶿ RHS - Eᶿ LHS/ Eᶿ oxidized- Eᶿ reduced
Substituting:
Overall Eᶿ = +0.80 – (- 0.13) = +0.93V
Overall redox equation:
2Ag+ (aq) + Pb(s) -> Pb2+ (aq) + 2Ag(s) Eᶿ = +0.93V
Overall conventional cell representation:
d)Chlorine and Bromine
From the table above:
Overall Eᶿ b>= Eᶿ higher- Eᶿ lower / Eᶿ RHS - Eᶿ LHS/ Eᶿ oxidized- Eᶿ reduced
Substituting:
Overall Eᶿ = - 0.13 – (- 1.36) = +1.23V
Overall redox equation:
Overall conventional cell representation:
Chlorine displaces bromine from bromine water. when Chlorine gas is thus bubbled in bromine water, the pale green colour fades as displacement takes place and a brown solution containing dissolved bromine liquid is formed.
this reaction is feasible /possible because the overall redox reaction has a positive Eᶿ value.
e)Strongest oxidizing agent and the strongest reducing agent.
From the table above:
Overall Eᶿ = Eᶿ higher- Eᶿ lower / Eᶿ RHS - Eᶿ LHS/ Eᶿ oxidized- Eᶿ reduced
Substituting:
Overall Eᶿ = +2.87 – (-2.92) = +5.79V
Overall redox equation:
Overall conventional cell representation:
2K(s) / 2K+ (aq),1M, // 1M, 2F- (aq) / F2(g) Eᶿ = +5.79V
the redox reactions in an electrochemical/voltaic is commercially applied to make the:
(a)Dry /primary/Laclanche cell.
(b)Wet /secondary /accumulators.
(a)Dry/primary/Laclanche cell
Examine a used dry cell.
Note the positive and the negative terminal of the cell. Carefully using a knife cut a cross section from one terminal to the other.
the dry cell consist of a Zinc can containing a graphite rod at the centre surrounded by a paste of;
-Ammonium chloride -Zinc chloride - Powdered manganese (IV) oxide mixed with Carbon.
Zinc acts/serve as the negative terminal where it ionizes/dissociates:
Ammonium ions in ammonium chloride serve as the positive terminal where it is converted to ammonia gas and hydrogen gas. 2NH4+(aq) + 2e -> 2NH3(g) + H2(g)
Ammonia forms a complex salt / compound /(Zn(NH3) 4)2+ (aq) / tetramminezinc(II) complex with the Zinc chloride in the paste.
Manganese (IV) oxide oxidizes the hydrogen produced at the electrodes to water preventing any bubbles from coating the carbon terminal which would reduce the efficiency of the cell.
Ammonium chloride is used as paste because the solid does not conduct electricity because the ions are fused/not mobile.
Since the reactants are used up, the dry /primary /Laclanche cell cannot provide continous supply of electricity.
The process of restoring the reactants is called recharging.
b)Wet/Secondary/Accumulators
1. Wet/Secondary/Accumulators are rechargeable unlike dry /primary /Laclanche cells.Wet/Secondary/Accumulators are made up of:
(i)Lead plate that forms the negative terminal
(ii)Lead(IV) oxide that forms the positive terminal
2.The two electrodes are dipped in concentrated Sulphuric(VI) acid of a relative density 1.2/1.3
3.At the negative terminal,lead ionizes /dissolves;
4.At the positive terminal,
(i) Lead(IV) oxide reacts with the hydrogen ions in Sulphuric(VI)acid to form Pb2+ (aq) ions;
(ii) Pb2+ (aq) ions formed instantly react with Sulphate (VI) ions/ SO42- (aq) from Sulphuric (VI)acid to form insoluble Lead(II) Sulphate (VI).
Pb2+ (aq) + SO42- (aq) -> PbSO4(s)
5.The overall cell reaction is called discharging
PbO2(s) +Pb(s) + 4H+(aq) + 2SO42- (aq)-> 2PbSO4(s) + 2H2O(l) Eᶿ = +2.0V
6.The insoluble Lead(II) Sulphate (VI) formed should not be left for long since fine Lead(II) Sulphate (VI) will Change to a course non-reversible and inactive form making the cell less efficient.
As the battery discharges ,lead and lead(IV)oxide are depleted/finished/reduced and the concentration of Sulphuric(VI)acid decreases.
7. During recharging, the electrode reaction is reversed as below:
(Iii)electrolysis (Electrolytic Cell)
1.Electrolysis is defined simply as the decomposition of a compound by an electric current/electricity.
A compound that is decomposed by an electric current is called an electrolyte. Some electrolytes are weak wHile others are strong.
2.Strong electrolytes are those that are fully ionized/dissociated into (many) ions.
Common strong electrolytes include:
(i)all mineral acids
(ii)all strong alkalis/sodium Hydroxide/potassium Hydroxide.
(iii)all soluble salts
3.Weak electrolytes are those that are partially/partly ionized/dissociated into (few) ions.
Common weak electrolytes include:
(i)all organic acids
(ii)all bases except sodium Hydroxide/potassium Hydroxide.
(iii)Water
4. A compound that is not decomposed by an electric current is called non-electrolyte.
Non-electrolytes are those compounds /substances that exist as molecules and thus cannot ionize/dissociate into(any) ions .
Common non-electrolytes include:
(i) most organic solvents (e.g. petrol/paraffin/benzene/methylbenzene/etHanol)
(ii)all Hydrocarbons(alkanes /alkenes/alkynes)
(iii)chemicals of life(e.g. proteins, carbohydrates, lipids, starch, sugar)
5. An electrolytes in solid state havefused /joined ions and therefore do not conduct electricity but the ions (cations and anions) are free and mobile in molten and aqueous (solution, dissolved in water) state.
6.During electrolysis, the free ions are attracted to the electrodes.
An electrode is a rod through which current enter and leave the electrolyte during electrolysis.
An electrode that does not influence/alter the products of electrolysis is called an inert electrode.
Common inert electrodes include:
(i)Platinum
(ii)Carbon graphite
Platinum is not usually used in a school laboratory because it is very expensive. Carbon graphite is easily/readily and cheaply available (from used dry cells).
7.The positive electrode is called Anode.
The anode is the electrode through which current enter the electrolyte/electrons leave the electrolyte 8.The negative electrode is called cathode.
the cathode is the electrode through which current leave the electrolyte / electrons enter the electrolyte
9. During the electrolysis, free anions are attracted to the anode where they lose /donate electrons to form neutral atoms/molecules. i.e.
the neutral atoms /molecules form the products of electrolysis at the anode. this is called discharge at anode
10. During electrolysis, free cations are attracted to the cathode where they gain /accept/acquire electrons to form neutral atoms/molecules.
X+ (aq) + 2e -> X(s) (for cations from electrolytes in aqueous state / solution / dissolved in water)
2X+ (l) + 2e -> X (l) (for cations from molten electrolytes) the neutral atoms /molecules form the products of electrolysis at the cathode.
this is called discharge at cathode.
11. the below set up shows an electrolytic cell.
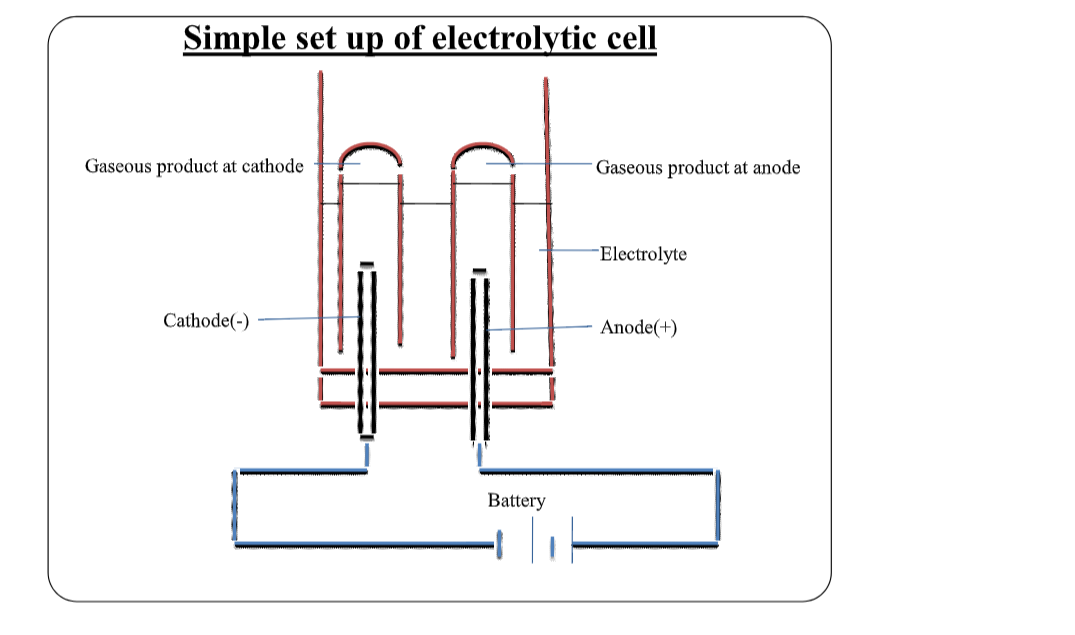
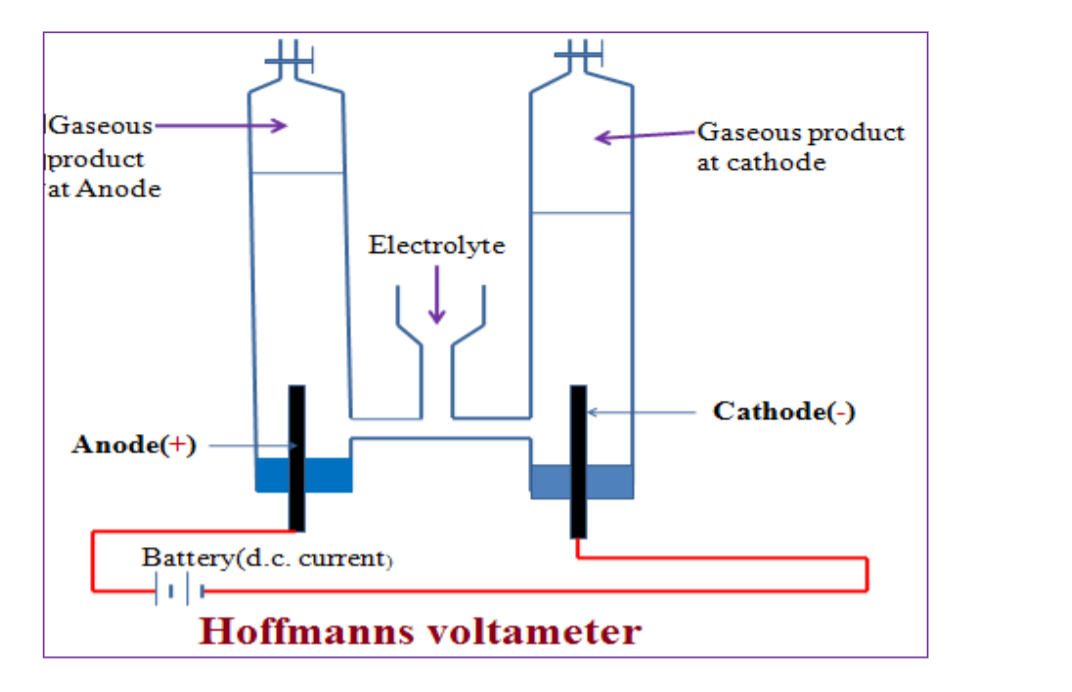
(i)Decomposition of electrolyte into free ions;
(ii)At the cathode/negative electrode(-);
(iii)At the anode/positive electrode(+);
(iv)Products of electrolysis therefore are;
I.At the cathode grey beads /solid lead metal.
II.At the anode pale green Chlorine gas.
b)To determine the products of electrolysis of molten Zinc bromide
(i)Decomposition of electrolyte into free ions;
(ii)At the cathode/negative electrode(-);
(Cation / Zn2+ gains / accepts / acquires electrons to form free atom)
(iii)At the anode/positive electrode(+);
2Br-(l) -> Br2 (g) + 2e
(Anion / Br- donate/lose electrons to form free atom then a liquid molecule which Change to gas on heating)
(iv)Products of electrolysis therefore are;
I.At the cathode grey beads /solid Zinc metal.
II.At the anode red bromine liquid / red/brown bromine gas.
c)To determine the products of electrolysis of molten sodium chloride
(i)Decomposition of electrolyte into free ions;
(Compound decomposed into free cation and anion in liquid state)
(ii)At the cathode/negative electrode(-);
(Cation / Na+ gains / accepts / acquires electrons to form free atom)
(iii)At the anode/positive electrode(+);
(iv)Products of electrolysis therefore are;
I.At the cathode grey beads /solid sodium metal.
II.At the anode pale green Chlorine gas.
d)To determine the products of electrolysis of molten Aluminium (III)oxide
(i)Decomposition of electrolyte into free ions;
(ii)At the cathode/negative electrode(-);
(iii)At the anode/positive electrode(+);
(Anion /6O2- donate/lose 12 electrons to form free atom then three gas molecule)
(iv)Products of electrolysis therefore are;
I.At the cathode grey beads /solid aluminium metal.
II.At the anode colourless gas that relights/rekindles glowing splint.
13. For a compound /salt mixture containing many ions in an electrolytic cell, the discharge of ions in the cell depend on the following factors:
a) Position of cations and anions in the electro chemical series
1. Most electro positive cations require more energy to reduce (gain electrons) and thus not readily discharged.
The higher elements /metals in the electro chemical series the less easily/readily it is discharged at the cathode in the electrolytic cell.
Table I showing the relative ease of discharge of cations in an electrolytic cell
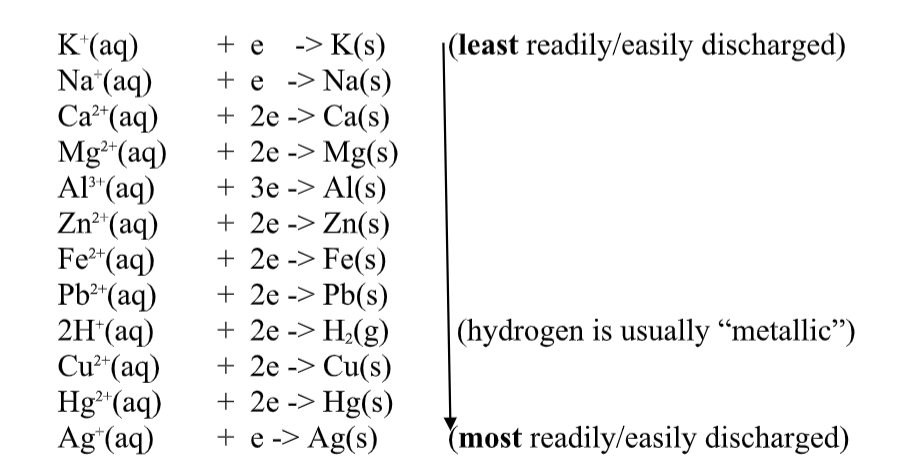
All the other anionic radicals(SO42- ,SO32- ,CO32- ,HSO4- ,HCO3- ,NO3- ,PO43-)are not/never discharged. the ease of discharge of Halogen ions increase down the group.
Table II showing the relative ease of discharge of anions in an electrolytic cell
4OH- (aq) -> 2H2O(l) + O2 (g) + 4e (most readily/easily discharged)
3.(a)when two or more cations are attracted to the cathode, the ion lower in the electrochemical series is discharged instead of that which is higher as per the table I above.
this is called selective/preferential discharge at cathode.
(b)when two or more anions are attracted to the anode, the ion higher in the electrochemical series is discharged instead of that which is lower as per the table I above. this is called selective/preferential discharge at anode.
4.The following experiments shows the influence /effect of selective/preferential discharge on the products of electrolysis:
(i)Electrolysis of acidified water/dilute Sulphuric(VI) acid
Fill the Hoffmann voltameter with dilute Sulphuric(VI) acid. Connect the Hoffmann voltameter to a d.c. electric supply.
Note the observations at eachelectrode.
Electrolytic cell set up during electrolysis of acidified water/dilute Sulphuric(VI) acid
Answer the following questions:
I. Write the equation for the decomposition of the electrolytes during the electrolytic process.
cathode- H+(aq) from either Sulphuric(VI) acid (H2 SO4) or water (H2O)
Anode- SO42-(aq) from Sulphuric (VI) acid (H2 SO4) and OH- (aq) from water (H2O)
III. Write the equation for the reaction during the electrolytic process at the:
IV. Name the products of electrolysis of acidified water.
cathode-hydrogen gas (colourless gas that extinguisHes burning splint with explosion/ “pop” sound
Anode-Oxygen gas (colourless gas that relights /rekindles glowing splint)
V. Explain the difference in volume of products at the cathode and anode.
the four(4) electrons donated/lost by OH- ions to form 1 molecule/1volume/1mole of oxygen (O2)gas at the anode are gained/acquired/accepted by the four H+(aq) ions to form 2 molecule/2volume/2mole of hydrogen (H2)gas at the cathode.
the volume of Oxygen gas at the anode is thus a half the volume of hydrogen produced at the cathode/ the volume of hydrogen gas at the cathode is thus a twice the volume of Oxygen produced at the anode.
VI. why is electrolysis of dilute Sulphuric(VI) acid called “electrolysis of (acidified) water”?
the ratio of H2 (g): O2 (g) is 2:1 as they are combined in water. this implies/means that water in the electrolyte is being decomposed into hydrogen and Oxygen gases.
the electrolysis of dilute Sulphuric acid is therefore called “electrolysis of acidified water.”
VI. Explain the changes in concentration of the electrolyte during electrolysis of acidified water”
the concentration of dilute Sulphuric (VI) acid increases.
Water in the electrolyte is decomposed into hydrogen and Oxygen gases that escape.
the concentration /mole of acid present in a given volume of solution thus continue increasing/rising.
(ii)Electrolysis of Magnesium Sulphate(VI) solution
Fill the Hoffmann voltameter with dilute Sulphuric(VI) acid.
Connect the Hoffmann voltameter to a d.c. electric supply. Note the observations at eachelectrode.
Answer the following questions:
I. Write the equation for the decomposition of the electrolytes during the electrolytic process.
Mg SO4(aq) -> SO42-(aq) + Mg2+(aq)
II. Name the ions in Magnesium Sulphate(VI) solution that are attracted/move to:
cathode- Mg2+(aq) from Magnesium Sulphate(VI) solution (Mg SO4) and H+(aq) from water (H2O)
Anode- SO42-(aq) from Magnesium Sulphate(VI) solution (Mg SO4) and OH- (aq) from water (H2O)
III. Write the equation for the reaction during the electrolytic process at the:
cathode
H+ ions selectively discharged instead of Mg2+ ions at the cathode)
Anode 4OH- (aq) -> 2H2O(l) + O2 (g) + 4e
(4OH- ions selectively discharged instead of SO42- ions at the anode)
IV. Name the products of electrolysis of Magnesium Sulphate(VI) solution
cathode-hydrogen gas (colourless gas that extinguisHes burning splint with explosion/ “pop” sound
Anode-Oxygen gas (colourless gas that relights /rekindles glowing splint)
V. Explain the difference in volume of products at the cathode and anode.
the four(4) electrons donated/lost by OH- ions to form 1 molecule/1volume/1mole of oxygen (O2)gas at the anode are gained/acquired/accepted by the four H+(aq) ions to form 2 molecule/2volume/2mole of hydrogen (H2)gas at the cathode.
the volume of Oxygen gas at the anode is thus a half the volume of hydrogen produced at the cathode/ the volume of hydrogen gas at the cathode is thus a twice the volume of Oxygen produced at the anode.
VI. Explain the changes in concentration of the electrolyte during electrolysis of Magnesium Sulphate(VI) solution
the concentration of dilute Magnesium Sulphate(VI) solution increases.
the ratio of H2 (g): O2 (g) is 2:1 as they are combined in water.
Water in the electrolyte is decomposed into hydrogen and Oxygen gases that escape as products.
The concentration /mole of acid present in a given volume of Magnesium Sulphate(VI) solution thus continue increasing/rising. the set – up below was used during the electrolysis of aqueous magnesium Sulphate using inert electrodes.
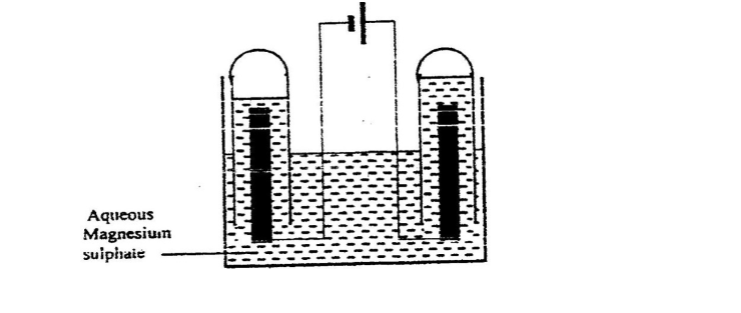
Identify the ions and cations in the solution
On the diagram label the cathode
Write ionic equations for the reactions that took place at the anode.
Explain the Change that occurred to the concentration of magnesium Sulphate solution during the experience.
During the electrolysis a current of 2 amperes was passed throughthe solution for 4 Hours.
Calculate the volume of the gas produced at the anode.(1 faraday 96500 coulombs and volume of a gas at room temperature is 24000cm3)
One of the uses of electrolysis is electroplating
what is meant by electroplating?
Give tow reasons why electroplating is necessary.
b) Concentration of the electrolytes
1.High concentrations of cations and/or anions at the electrodes block the ion/s that is likely to be discharged at the electrode.
This is called over voltage.
A concentrated solution therefore produces different products of electrolysis from a dilute one.
2. the following experiments show the influence/effect of concentration of electrolyte on the products of electrolysis.
(i)Electrolysis of dilute and concentrated(brine)sodium chloride solution
I. Dissolve about 0.5 g of pure sodium chloride crystals in 100cm3 of water. Place the solution in an electrolytic cell. Note the observations at eachelectrode for 10 minutes. Transfer the set up into a fume chamber/open and continue to make observations for a further 10 minute.
Answer the following questions:
I. Write the equation for the decomposition of the electrolytes during the electrolytic process.
H2O(l) -> OH- (aq) + H+(aq)
NaCl(aq) -> Cl-(aq) + Na+(aq)
II. Name the ions in sodium chloride solution that are attracted/move to:
cathode- Na+(aq) from Sodium chloride solution (NaCl) and H+(aq) from water (H2O)
Anode- Cl-(aq) from sodiumchloride solution (NaCl) and OH- (aq) from water (H2O)
III. Write the equation for the reaction during the electrolytic process at the:
cathode 4H+(aq) + 4e -> 2H2(g)
H+ ions selectively discharged instead of Na+ ions at the cathode)
Anode 4OH- (aq) -> 2H2O(l) + O2 (g) + 4e
(4OH- ions selectively discharged instead of Cl- ions at the anode)
IV. Name the products of electrolysis of dilute sodium chloride solution
cathode-hydrogen gas (colourless gas that extinguishes burning splint with explosion/ “pop” sound
Anode-Oxygen gas (colourless gas that relights /rekindles glowing splint)
V. Explain the difference in volume of products at the cathode and anode.
Four(4) electrons donated/lost by OH- ions to form 1 molecule/1volume/1mole of oxygen (O2)gas at the anode are gained/acquired/accepted by four H+(aq) ions to form 2 molecule/2volume/2mole of hydrogen (H2)gas at the cathode.
the volume of Oxygen gas at the anode is half the volume of hydrogen produced at the cathode/ the volume of hydrogen gas at the cathode is twice the volume of Oxygen produced at the anode.
VI. Explain the changes in concentration of the electrolyte during electrolysis of sodium chloride solution
the concentration of dilute sodium chloride solution increases.
the ratio of H2 (g): O2 (g) is 2:1 as they are combined in water. Water in the electrolyte is decomposed into hydrogen and Oxygen gases that escape as products. The concentration /moles of salt present in a given volume of sodium chloride solution continue increasing/rising.
II. Dissolve about 20 g of pure sodium chloride crystals in 100cm3 of water. Place the solution in an electrolytic cell. Note the observations continuously at eachelectrode for 30 minutes in a fume chamber/open.
Answer the following questions:
I. Write the equation for the decomposition of the electrolytes during the electrolytic process.
H2O(l) -> OH- (aq) + H+(aq)
NaCl(aq) -> Cl-(aq) + Na+(aq)
II. Name the ions in sodium chloride solution that are attracted/move to:
cathode- Na+(aq) from Sodium chloride solution (NaCl) and H+(aq) from water (H2O)
Anode- Cl-(aq) from sodium chloride solution (NaCl) and OH- (aq) from water (H2O)
III. Write the equation for the reaction during the electrolytic process at the:
cathode 2H+(aq) + 2e -> H2(g)
H+ ions selectively discharged instead of Na+ ions at the cathode)
Anode 2Cl- (aq) -> Cl2(g) + 4e
(Cl- ions with a higher concentration block the discharge of OH- ions at the anode)
IV. Name the products of electrolysis of concentrated sodium chloride solution/brine
cathode-hydrogen gas (colourless gas that extinguishes burning splint with explosion/ “pop” sound
Anode-Chlorine gas(pale green gas that bleaches damp/moist/wet litmus papers)
V. Explain the difference in volume of products at the cathode and anode.
Two (2) electrons donated/lost by Cl- ions to form 1 molecule/1volume/1mole of Chlorine (Cl2)gas at the anode are gained/acquired/accepted by two H+(aq) ions to form 1 molecule/1volume/1mole of hydrogen (H2)gas at the cathode.
the volume of Chlorine gas at the anode is equal to the volume of hydrogen produced at the cathode/ the volume of hydrogen gas at the cathode is equal to the volume of Chlorine produced at the anode.
VI. Explain the changes in concentration of the electrolyte during electrolysis of concentrated sodium chloride solution/brine
the concentration of concentrated sodium chloride solution/brine increases.
the ratio of Cl2 (g): H2 (g) is 1:1 as they are combined in water.
Water in the electrolyte is decomposed into only hydrogen gas that escapes as products at cathode.
the concentration /moles of OH- (aq) and Na+ ion (as NaOH) present in a given volume of electrolyte continue increasing/rising.
thismakes the electrolyte strongly alkaline with High pH.
As the electrolysis of brine continues the concentration of Cl- ions decrease and oxygen gas start being liberated at anode.
the electrolyte pH is thus lowered and the concentration of brine starts again increasing.
(ii)Electrolysis of dilute and concentrated Hydrochloric acid solution
I. Prepare about 50cm3 of 0.05 M of dilute Hydrochloric acid in 100cm3 solution. Place the solution in an electrolytic cell. Note the observations at eachelectrode for 10 minutes.
Answer the following questions:
I. Write the equation for the decomposition of the electrolytes during the electrolytic process.
H2O(l) -> OH- (aq) + H+(aq)
HCl(aq) -> Cl-(aq) + H+(aq)
II. Name the ions in dilute Hydrochloric acid solution that are attracted/move to:
cathode- H+(aq) from dilute Hydrochloric acid (HCl) and H+(aq) from water (H2O)
Anode- Cl-(aq) from dilute Hydrochloric acid (HCl) and OH- (aq) from water (H2O)
III. Write the equation for the reaction during the electrolytic process at the:
cathode 4H+(aq) + 4e -> 2H2(g)
H+ ions selectively discharged instead of Na+ ions at the cathode)
Anode 4OH- (aq) -> H2O(l) +O2+ 4e
(4OH- ions selectively discharged instead of Cl- ions at the anode)
IV. Name the products of electrolysis of dilute Hydrochloric acid
cathode-hydrogen gas (colourless gas that extinguisHes burning splint with explosion/ “pop” sound
Anode-Oxygen gas (colourless gas that relights /rekindles glowing splint)
V. Explain the difference in volume of products at the cathode and anode.
Four(4) electrons donated/lost by OH- ions to form 1 molecule/1volume/1mole of oxygen (O2)gas at the anode are gained/acquired/accepted by four H+(aq) ions to form 2 molecule/2volume/2mole of hydrogen (H2)gas at the cathode.
the volume of Oxygen gas at the anode is half the volume of hydrogen produced at the cathode/ the volume of hydrogen gas at the cathode is twice the volume of Oxygen produced at the anode.
VI. Explain the changes in concentration of the electrolyte during electrolysis of dilute Hydrochloric acid
the concentration of dilute Hydrochloric acid increases.
the ratio of H2 (g): O2 (g) is 2:1 as they are combined in water. Water in the electrolyte is decomposed into hydrogen and Oxygen gases that escape as products. the concentration /moles of HCl present in a given volume of dilute Hydrochloric acid continue increasing/rising.
II. Prepare about 50cm3 of 2M of Hydrochloric acid in 100cm3 solution. Place the solution in an electrolytic cell. Note the observations at eachelectrode for 30 minutes
Caution this experiment should be done in the open/fume chamber.
Answer the following questions:
I. Write the equation for the decomposition of the electrolytes during the electrolytic process.
H2O(l) -> OH- (aq) + H+(aq)
HCl(aq) -> Cl-(aq) + H+(aq)
II. Name the ions in 2M Hydrochloric acid solution that are attracted/move to:
cathode- H+(aq) from dilute Hydrochloric acid (HCl) and H+(aq) from water (H2O)
Anode- Cl-(aq) from dilute Hydrochloric acid (HCl) and OH- (aq) from water (H2O)
III. Write the equation for the reaction during the electrolytic process at the:
cathode 4H+(aq) + 4e -> 2H2(g)
H+ ions selectively discharged instead of Na+ ions at the cathode)
Anode 2Cl- (aq) -> Cl2+ 2e
(OH- ions concentration is low.Cl- ions concentration is higher at the anode thus cause over voltage/block discharge of OH- ions)
IV. Name the products of electrolysis of 2M Hydrochloric acid
cathode-hydrogen gas (colourless gas that extinguisHes burning splint with explosion/ “pop” sound
Anode-Chlorine gas (Pale green gas that bleaches blue/red moist/wet/damp litmus papers)
V. Explain the difference in volume of products at the cathode and anode.
Two(2) electrons donated/lost by Cl- ions to form 1 molecule/1volume/1mole of Chlorine (Cl2)gas at the anode are gained/acquired/accepted by two H+(aq) ions to form 1 molecule/1volume/1mole of hydrogen (H2)gas at the cathode.
the volume of Chlorine gas at the anode is equal to the volume of hydrogen produced at the cathode/ the volume of hydrogen gas at the cathode is twice the volume of Chlorine produced at the anode.
VI. Explain the changes in concentration of the electrolyte during electrolysis of 2M Hydrochloric acid
the concentration of Hydrochloric acid decreases.
the ratio of H2 (g): Cl2 (g) is 1:1 as they are combined in Hydrochloric acid.
Water in the electrolyte is decomposed only into hydrogen gas that escapes as products at the cathode.
there is a net accumulation of excess OH- (aq) ions in solution.
this makes the electrolyte strongly alkaline with High pH.
c) Nature of electrodes used in the electrolytic cell
Inert electrodes (carbon-graphite and platinum) do not alter the expected products of electrolysis in an electrolytic cell. If another/different electrode is used in the electrolytic cell it alters/influences/changes the expected products of electrolysis.
The examples below illustrate the influence of the nature of electrode on the products of electrolysis:
(i)Electrolysis of copper(II) Sulphate(VI) solution
I. Using carbon-graphite electrodes
Weigh Carbon -graphite electrodes. Record the masses of the electrodes in table I below. Place the electrodes in 1M copper(II) Sulphate(VI) solution in a beaker. Set up an electrolytic cell.
Close the switch and pass current for about 20 minutes. Observe eachelectrode and any changes in electrolyte. Remove the electrodes from the electrolyte. Wash with acetone/propanone and allow them to dry. Reweigh eachelectrode.
Sample results
I. Write the equation for the decomposition of the electrolytes during the electrolytic process.
H2O(l) -> OH- (aq) + H+(aq)
CuSO4(aq) -> SO42-(aq) + Cu2+(aq)
II. Name the ions in 1M copper(II) Sulphate(VI) solution that are attracted/move to:
cathode- Cu2+ (aq) from copper(II) Sulphate(VI) solution and H+(aq) from water (H2O)
Anode- SO42-(aq) from copper(II) Sulphate(VI) solution and OH- (aq) from water (H2O)
III. Write the equation for the reaction during the electrolytic process at the:
cathode 2Cu2+ (aq) + 4e -> 2Cu(g)
Cu2+ ions are lower than H+ ions in the electrochemical series therefore selectively discharged at the cathode.)
Anode 4OH- (aq) -> H2O(l) + O2+ 4e
(OH- ions ions are higher than SO42- ions in the electrochemical series therefore selectively discharged at the cathode.))
IV. Name the products of electrolysis of 1M copper(II) Sulphate(VI) solution
cathode-2 moles of copper metal as brown solid coat
Anode-Oxygen gas (Colourless gas that relights /rekindles glowing splint)
V. Explain the changes that take place at the cathode and anode.
Four(4) electrons donated/lost by OH- ions to form 1 molecule/1volume/1mole of Oxygen (O2)gas at the anode are gained/acquired/accepted by two Cu2+(aq) ions to form 2 moles of brown copper solid that deposit itself at the cathode.
The moles of oxygen gas at the anode is equal to the moles of copper produced at the cathode
VI. Explain the changes in electrolyte during electrolysis of 1M copper (II) Sulphate(VI) solution.
(i)the pH of copper(II) Sulphate(VI) solution lowers/decreases. The salt becomes more acidic. Water in the electrolyte is decomposed only into Oxygen gas (from the OH- ions) that escapes as products at the anode. There is a net accumulation of excess H+ (aq) ions in solution. thismakes the electrolyte strongly acidic with low pH.
(ii) Cu2+ (aq) ions are responsible for the blue colour of the electrolyte/ copper(II) Sulphate (VI) solution. As electrolysis continues, blue Cu2+ (aq) ions gain electrons to form brown Copper. The blue colour of electrolyte therefore fades/become less blue.
(iii) Copper is deposited at the cathode. This increases the mass of the cathode.OH- ions that produce Oxygen gas at anode come from water. Oxygen escapes out/away without increasing the mass of anode.
II. Using copper electrodes
Weigh clean copper plates electrodes. Record the masses of the electrodes in table I below. Place the electrodes in 1M copper(II) Sulphate(VI) solution in a beaker. Set up an electrolytic cell.
Close the switch and pass current for about 20 minutes. Observe eachelectrode and any changes in electrolyte. Remove the electrodes from the electrolyte. Wash with acetone/propanone and allow them to dry. ReWeigh eachelectrode.
Sample results
I. Write the equation for the decomposition of the electrolytes during the electrolytic process.
H2O(l) -> OH- (aq) + H+(aq)
CuSO4(aq) -> SO42-(aq) + Cu2+(aq)
II. Name the ions in 1M copper(II) Sulphate(VI) solution that are attracted/move to:
cathode- Cu2+ (aq) from copper(II) Sulphate(VI) solution and H+(aq) from water (H2O)
Anode- SO42-(aq) from copper(II) Sulphate(VI) solution and OH- (aq) from water (H2O)
III. Write the equation for the reaction during the electrolytic process at the:
cathode Cu2+ (aq) + 2e -> Cu(s)
Cu2+ ions are lower than H+ ions in the electrochemical series therefore selectively discharged at the cathode.)
Anode Cu (s) -> Cu2+(aq) + 2e
(both OH- ions and SO42- ions move to the anode but none is discharged. the copper anode itself ionizes/dissolves/dissociate because less energy is used to remove an electron/ionize /dissociate copper atoms than OH- ions.
IV. Name the products of electrolysis of 1M copper(II) Sulphate(VI) solution using copper electrodes.
cathode-1 moles of copper metal as brown solid coat (cathode increase/deposits)
Anode-Anode erodes/decrease in size
V. Explain the changes that take place during the electrolytic process
(i)cathode
-Cu2+ ions are lower than H+ ions in the electrochemical series therefore selectively discharged at the cathode. Cu2+ ions havegreater tendency to accept/gain/acquire electrons to form brown copper atoms/solid that deposit itself and increase the mass/size of the cathode. The copper deposited at the cathode is pure
-H+ ions accumulate around the cathode. Electrolyte thus becomes strongly acidic around the cathode.
-Cu2+ ions in solution are responsible for the blue colour of electrolyte. Blue colour of electrolyte fade around the cathode.
(ii)Anode
Copper atom at the anode easily ionizes to release electrons. the anode therefore keeps decreasing in mass/eroding. the amount of copper that dissolve/erode is equal to the mass of copper deposited. this is called electrode ionization.
Electrode ionization is where the anode erodes/decrease and the cathode deposits/increase during electrolysis. the overall concentration of the electrolyte remains constant
14.In industries electrolysis has the following uses/applications:
(a)Extraction of reactive metals from their ores.
Potassium, sodium ,magnesium, and aluminium are extracted from their ores using electrolytic methods.
(b)Purifying copper after exraction from copper pyrites ores.
Copper obtained from copper pyrites ores is not pure. After extraction, the copper is refined by electrolysing copper(II)Sulphate(VI) solution using the impure copper as anode and a thin strip of pure copper as cathode. Electrode ionization take place there:
(i)At the cathode; Cu2+ (aq) + 2e -> Cu(s) (Pure copper deposits on the strip
(ii)At the anode; Cu(s) ->Cu2+ (aq) + 2e (impure copper erodes/dissolves)
(c)Electroplating
the label EPNS(Electro Plated Nickel Silver) on some steel/metallic utensils mean they are plated/coated with silver and/or Nickel to improve their appearance(add their aesthetic value)and prevent/slow corrosion(rusting of iron). Electroplating is the process of coating a metal with another metal using an electric current. During electroplating,the cathode is made of the metal to be coated/impure.
Example:
During the electroplating of a spoon with silver
(i)the spoon/impure is placed as the cathode(negative terminal of battery)
(ii)the pure silver is placed as the anode(positive terminal of battery)
(iii)the pure silver erodes/ionizes/dissociates to release electrons:
Ag(s) ->Ag+ (aq) + e (impure silver erodes/dissolves)
(iv) silver (Ag+)ions from electrolyte gain electrons to form pure silver deposits / coat /cover the spoon/impure
Ag+ (aq) + e ->Ag(s) (pure silver deposits /coat/cover on spoon)
Faradays 1st law of electrolysis states that “the mass/amount of substance liberated/produced/used during electrolysis is directly proportional to the quantity of of electricity passed/used.”
(a)the SI unit of quantity of electricity is the coulomb(C). the coulomb may be defined as the quantity of electricity passed/used when a current of one ampere flow for one second.i.e;
1Coulomb = 1 Ampere x 1Second
the Ampere is the SI unit of current(I)
the Second is the SI unit of time(t) therefore;
Quantity of electricity(in Coulombs) = Current(I) x time(t)
Practice examples
1. A current of 2 amperes was passed throughan electrolytic cell for 20 minutes. Calculate the quantity of electric Charge produced.
Working:
Quantity of electricity(in Coulombs) = Current(I) x time(t)
Substituting /converting time to second = 2 x (20 x 60)
= 2400 C
2. A current of 2 amperes was passed through an electrolytic.96500 coulombs of Charge were produced. Calculate the time taken.
Working:
Time(t) in seconds = Quantity of electricity(in Coulombs)
Current(I) in amperes
Substituting = 96500
2
= 48250 seconds
3. 96500 coulombs of Charge were produced after 10 minutes in an electrolytic cell . Calculate the amount of current used.
Working:
Current(I) in amperes = Quantity of electricity(in Coulombs) Time(t) in seconds
Substituting/converting time to second= 96500
10 x 60
= 160.8333 Amperes
(b)the quantity of electricity required for one mole of electrons at the anode/cathode is called the Faraday constant(F). It is about 96500 Coulombs.i.e
the number of Faradays used /required is equal to the number of electrons used at cathode/anode during the electrolytic process. e.g.
Cu2+ require to gain 2 moles of electrons=2 Faradays =2 x 96500 coulombs of electricity at the cathode.
Al3+ require to gain 3 moles of electrons=3 Faradays =3 x 96500 coulombs of electricity at the cathode
Na+ require to gain 1 moles of electrons=1 Faradays =1 x 96500 coulombs of electricity at the cathode
2H+ require to gain 2 moles of electrons=2 Faradays =2 x 96500 coulombs of electricity at the cathode to form 1molecule of hydrogen gas
2O2- require to lose/donate 4 moles of electrons=4 Faradays =4 x 96500 coulombs of electricity at the anode to form 1molecule of Oxygen O2 gas.
4OH- require to lose/donate 4 moles of electrons=4 Faradays =4 x 96500 coulombs of electricity at the anode to form 1molecule of Oxygen gas and 2 molecules of water.
(c)the mass/amount of products at the cathode/anode is related to the molar mass of the substance and/or the volume of gases at standard/room temperature and pressure as in the below examples:
Practice examples
1.Calculate the mass of copper deposited at the cathode when a steady current of 4.0 amperes is passed throughcopper(II)Sulphate(VI) for 30 minutes in an electrolytic cell. (Cu=63.5, 1F = 96500C)
Working:
Quantity of electricity(in Coulombs) = Current(I) x time(t)
Substituting /converting time to second = 4 x (30 x 60)
= 7200 C
Equation at the cathode: Cu2+ (aq) + 2e -> Cu(s)
2 mole of electrons = 2 Faradays = 2 x 96500 C produce a mass =molar mass of copper thus;
2 x 96500C -> 63.5 g
72000C -> 7200 x 63.5 = 2.3689 g of copper
2 x 96500
2.a)If 3.2 g of Lead were deposited when a current of 2.5 amperes was passed throughan electrolytic cell of molten Lead(II)bromide for 20 minutes, determine the Faraday constant.(Pb = 207)
Working:
Quantity of electricity (in Coulombs) = Current(I) x time(t)
Substituting /converting time to second = 2.5 x (20 x 60)
= 3000 C
If 3.2g of Lead -> 3000C
then 207 g of Lead -> 207 x 3000 = 194062.5 C
3.2
Equation at the cathode: Pb2+ (l) + 2e -> Pb(l)
From the equation: 2 moles of electrons = 2 Faradays = 194062.5 C
1mole of electrons = 1 Faraday => 194062.5 = 97031.25 C
2
b)what is the volume of bromine vapour produced at the anode at room temperature(1mole of gas at room temperature and pressure = 24000cm3)
Method 1
Equation at the anode: Br- (l) -> Br2(g) + 2e
From the equation: 2 moles of electrons = 2 Faradays = 194062.5 C -> 24000cm3
3000 C -> 3000 x 24000
194062.5
=371.0145cm3
Method 2
Equation at the anode: Br- (l) -> Br2(g) + 2e
Mole ratio of products at cathode: anode = 1:1
Moles of Lead at cathode = 3.2 = 0.0155moles = moles of Bromine
207
1 moles of bromine vapour -> 24000cm3
0.0155moles of Bromine -> 0.0155 x 24000 = 372 cm3
Method 3
Equation at the anode: Br- (l) -> Br2(g) + 2e
Ratio of Faradays used to form products at cathode: anode = 2:2
=> 2 x 97031.25 C produce 24000cm3 of bromine vapour
then: 3000 C -> 3000 x 24000cm3 = 371.0145cm3
2 x 97031.25
3.what mass of copper remain from 2.0 at the anode if a solution of copper(II)Sulphate(VI) is electrolysed using a current of 1 ampere flowing throughan electrolytic cell for 20 minutes.(Cu= 63.5, 1Faraday = 96487 coulombs)
Working:
Quantity of electricity (in Coulombs) = Current(I) x time(t)
Substituting /converting time to second = 1 x (20 x 60)
= 1200 C
Equation at the cathode: Cu2+ (aq) + 2e -> Cu(s)
2 mole of electrons = 2 Faradays = 2 x 96500 C erode/dissolve a mass =molar mass of copper thus;
2 x 96500C -> 63.5 g
1200C -> 1200 x 63.5 = 0.3948g of copper deposited
2 x 96500
Mass of copper remaining = Original mass – mass dissolved/eroded
=> 2.0 -0.3948 = 1.6052 g of copper remain
4. Calculate the current passed if a mass of 0.234 g of copper is deposited in 4 minutes during electrolysis of a solution of copper (II)Sulphate(VI).
(Cu= 63.5 ,1F = 96500C)
Working:
Equation at the cathode: Cu(s) -> Cu2+ (aq) + 2e
2 mole of electrons = 2 Faradays = 2 x 96500 C produce a mass =molar mass of copper thus;
63.5 g -> 2 x 96500C
0.234 g -> 0.234 x 2 x 96500 = 711.2126 C
63.5
Current(I) in amperes = Quantity of electricity(in Coulombs) Time(t) in seconds
Substituting/converting time to second= 711.2126 C
4x 60
= 2.9634 Amperes
5. (a) what quantity of electricity will deposit a mass of 2.43 g of Zinc during electrolysis of a solution of Zinc (II)Sulphate(VI).
(Zn= 65 ,1F = 96500C)
Working:
Equation at the cathode: Zn2+ (aq) + 2e -> Zn(s)
2 mole of electrons = 2 Faradays = 2 x 96500 C erode/dissolve a mass =molar mass of Zinc thus;
65 g -> 2 x 96500
2.43 g -> 2.43 x 2 x 96500 = 7215.2308 C
65
(b)Calculate the time (in minutes) it would take during electrolysis of the solution of Zinc (II)Sulphate(VI) above if a current of 4.0 Amperes is used.
Time(t) in seconds = Quantity of electricity(in Coulombs)
Current(I) in amperes
Substituting = 7215.2308 = 1803.8077 seconds = 30.0635 minutes
4 60
6.when a current of 1.5 amperes was passed througha cell containing M3+ ions of metal M for 15 minutes, the mass at cathode increased by 0.26 g.(Faraday constant = 96500C
a) Calculate the quantity of electricity used.
Quantity of electricity (in Coulombs) = Current(I) x time(t)
Substituting /converting time to second = 1.5 x (15 x 60)
= 1350 C
b) Determine the relative atomic mass of metal M
Equation at the cathode: M3+ (aq) + 3e -> M(s)
1350 C of electricity -> 0.26 g of metal M
3 mole of electrons = 3 Faradays = 3 x 96500 C produce a mass =molar mass of M thus;
RAM of M = 0.26 g x 3 x 96500 = 55.7556(No units)
1350
7.An element “P” has a relative atomic mass 88.when a current of 0.5 amperes was passed throughfused chloride of “P” for 32 minutes and 10seconds ,0.44 g of “P” was deposited at the cathode. Determine the Charge on an ion of “P”(Faraday constant = 96500C)
Working:
Quantity of electricity (in Coulombs) = Current(I) x time(t)
Substituting /converting time to second = 0.5 x ((32 x 60) + 10)
= 965C
0.44 g of metal “P” are deposited by 965C
88g of of metal “P” are deposited by: 88 x 965= 193000 C
0.44
96500 C = 1 mole of electrons = 1 Faradays = single Charge
193000 C -> 193000 = 2 moles/Faradays/Charges => symbol of ion = P2+
96500
8. During purification of copper by electrolysis 1.48 g of copper was deposited when a current was passed through aqueous copper (II)Sulphate(VI) for 2 ½ Hours. Calculate the amount of current that was passed. (Cu= 63.5 ,1F = 96500C)
Working:
Equation at the cathode: Cu2+ (aq) + 2e-> Cu(s)
2 mole of electrons = 2 Faradays = 2 x 96500 C produce a mass =molar mass of copper thus;
63.5 g -> 2 x 96500C
1.48 g -> 1.48 x 2 x 96500 = 4255.1181 C
63.5
Current(I) in amperes = Quantity of electricity(in Coulombs) Time(t) in seconds
Substituting/converting time to second= 4255.1181C
(( 2 x 60) + 30) x60
= 0.4728 Amperes
17. Practically Faraday 1st law of electrolysis can be verified as below.
Verifying Faraday 1st law of electrolysis
Procedure.
Weigh clean copper plates electrodes. Record the masses of the electrodes in table I below. Place the electrodes in 1M copper(II) Sulphate(VI) solution in a beaker. Set up an electrolytic cell.
Close the switch and pass a steady current of 2 amperes by adjusting the rheostat for exactly 20 minutes. Remove the electrodes from the electrolyte. Wash with acetone/ propanone and allow them to dry. ReWeigh eachelectrode.
Sample results
I. Write the equation for the decomposition of the electrolytes during the electrolytic process.
H2O(l) -> OH- (aq) + H+(aq)
CuSO4(aq) -> SO42-(aq) + Cu2+(aq)
II. Name the ions in 1M copper(II) Sulphate(VI) solution that are attracted/move to:
cathode- Cu2+ (aq) from copper(II) Sulphate(VI) solution and H+(aq) from water (H2O)
Anode- SO42-(aq) from copper(II) Sulphate(VI) solution and OH- (aq) from water (H2O)
III. Write the equation for the reaction during the electrolytic process at the:
cathode Cu2+ (aq) + 2e -> Cu(s)
Cu2+ ions are lower than H+ ions in the electro chemical series therefore selectively discharged at the cathode.)
Anode Cu (s) -> Cu2+(aq) + 2e
(both OH- ions and SO42- ions move to the anode but none is discharged. The copper anode itself ionizes/dissolves/dissociate as less energy is used to remove an electron/ionize /dissociate copper atoms than OH- ions.
IV. Name the products of electrolysis of 1M copper(II) Sulphate(VI) solution using copper electrodes.
cathode-1.25 g of copper metal as brown solid coat/deposits
Anode-1.25 g of copper metal erodes/decrease in size
V. (i)How many moles of electrons are used to deposit/erode one mole of copper metal at the cathode/anode?
From the equation at anode/cathode= 2 moles
(ii)How many Faradays are used to deposit/erode one mole of copper metal at the cathode/anode?
From the equation at anode/cathode : 2 moles = 2 Faradays
(iii)Calculate the quantity of electric Charge used
Working:
Quantity of electricity (in Coulombs) = Current(I) x time(t)
Substituting /converting time to second = 2 x 20 x 60
= 2400C
VI. (i) Calculate the quantity of electricity required to deposit/erode one mole of copper at the cathode/anode(Cu=63.5)
Since 1.25 g of copper -> 2400C
then 63.5 g (1mole of copper) -> 63.5 x 2400 = 121920 C
1.25
(ii)Determine the Faraday constant from the results in V(i) above
From the equation at;
cathode Cu2+ (aq) + 2e -> Cu(s)
Anode Cu (s) -> Cu2+(aq) + 2e
2 moles = 2 Faradays -> 121920 C
1 moles = 1 Faradays -> 121920/2 = 60960 C
(iii) the faraday constant obtained above is far lower than theoretical. Explain
-High resistance of the wires used.
- Temperatures at 25oc were not kept constant
- Plates/electrodes used were not made of pure copper
- Plates/electrodes used were not thoroughly clean copper
Further practice
1.An element P has a relative atomic mass of 88. when a current of 0.5 amperes was passed through the fused chloride of P for 32 minutes and 10 seconds, 0.44g of P were deposited at the cathode. Determine the Charge on an ion of P. (1 faraday = 96500 Coulombs).
2.During electrolysis of aqueous copper (II) Sulphate, 144750 coulombs of electricity were used. Calculate the mass of copper metal that was obtained
(Cu = 64 ;1 Faraday = 96500 coulombs) ( 3 mks)
3.A nitrate of a metal M was electrolysed .1.18 g of metal was deposited when a current of 4 ampheres flow for 16 minutes.Determine the formula of the Sulphate(VI)salt of the metal.
(Faraday constant = 96500 , RAM of X = 59.0)
Initial mass of cathode = 1.0 g
Final mass of cathode = 1.6 g
Change in mass of cathode = 0.60 g
(i)Determine the Change in mass at the anode. Explain your answer.
KCSE Revision Notes Form 1 - Form 4 All Subjects
Basic Chemistry Interview Questions and Answers Pdf
Basic Chemistry Questions and Answers
Basic Chemistry Questions and Answers Pdf
Chemistry Form 1 Notes
Chemistry Form 1 Notes Free Download
Chemistry Form 1 Notes Pdf
Chemistry Form 1 Notes Pdf Download
Chemistry Form 2 Notes
Chemistry Form 2 Questions and Answers
Chemistry Form 2 Salts
Chemistry Form 2 Structure and Bonding
Chemistry Form 3 Notes
Chemistry Form 3 Notes Pdf
Chemistry Form 4 Exercise
Chemistry Form 4 Notes
Chemistry Form 4 Notes Chapter 1
Chemistry Form 4 Notes Chapter 2
Chemistry Form 4 Notes Chapter 3
Chemistry Form 4 Notes Download
Chemistry Form 4 Notes Pdf
Chemistry Form One Book Pdf
Chemistry Form One Notes Pdf
Chemistry Form One Pdf
Chemistry Multiple Choice Questions and Answers Pdf
Chemistry Objective Questions for Competitive Exams
Chemistry Question and Answer With Explanation
Chemistry Questions and Answers
Chemistry Questions and Answers for High School
Chemistry Questions and Answers Pdf
Chemistry Questions and Answers Pdf for Class 12
Chemistry Quiz Questions and Answers for Class 12
Chemistry Quiz Questions and Answers Multiple Choice
Chemistry Spm Notes Download
Download Chemistry Form 2 Notes
Download Klb Chemistry Book 3
Form 3 Chemistry Questions and Answers
Form 3 Chemistry Questions and Answers Pdf
Form Three Chemistry Book Pdf
Hard Chemistry Questions and Answers
High School Chemistry Questions and Answers Pdf
Inorganic Chemistry Multiple Choice Questions With Answers Pdf
Inorganic Chemistry Questions and Answers Pdf
Interesting Chemistry Questions
Klb Chemistry Book 1 Download
Klb Chemistry Book 1 Pdf
Klb Chemistry Book 2 Notes
Klb Chemistry Book 2 Notes Pdf
Klb Chemistry Book 3 Notes
Klb Chemistry Form 2 Notes
Klb Chemistry Form 2 Pdf
Klb Chemistry Form 3 Pdf
Spm Chemistry Revision Notes
Tricky Chemistry Questions With Answers
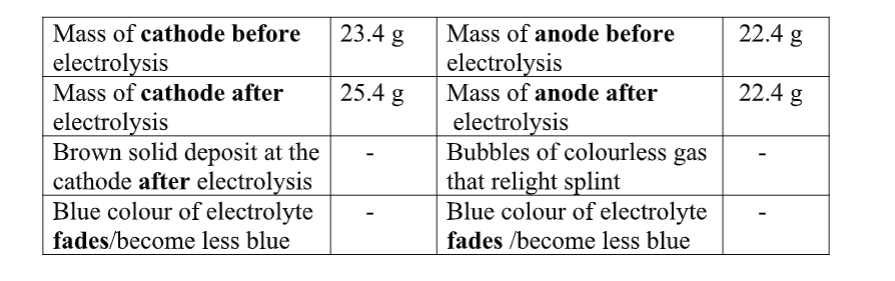
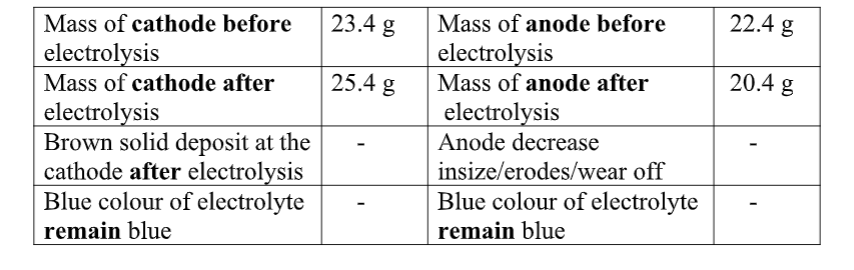
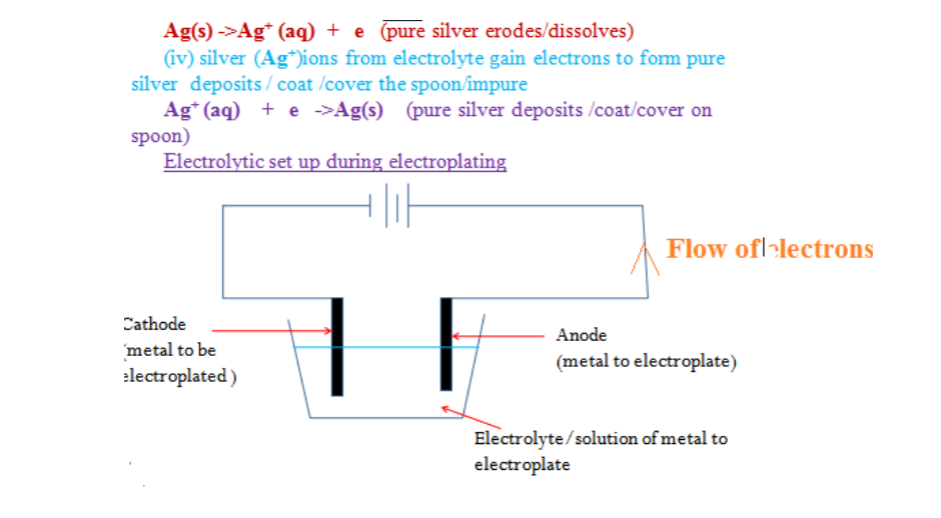


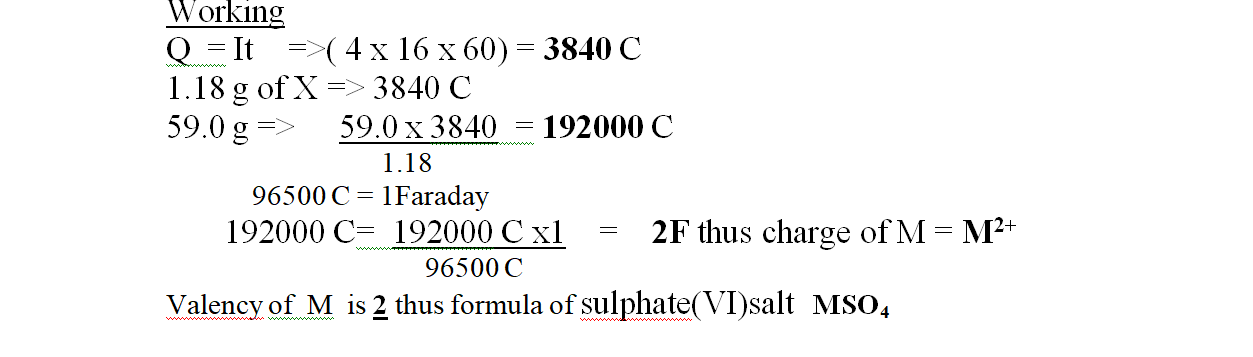
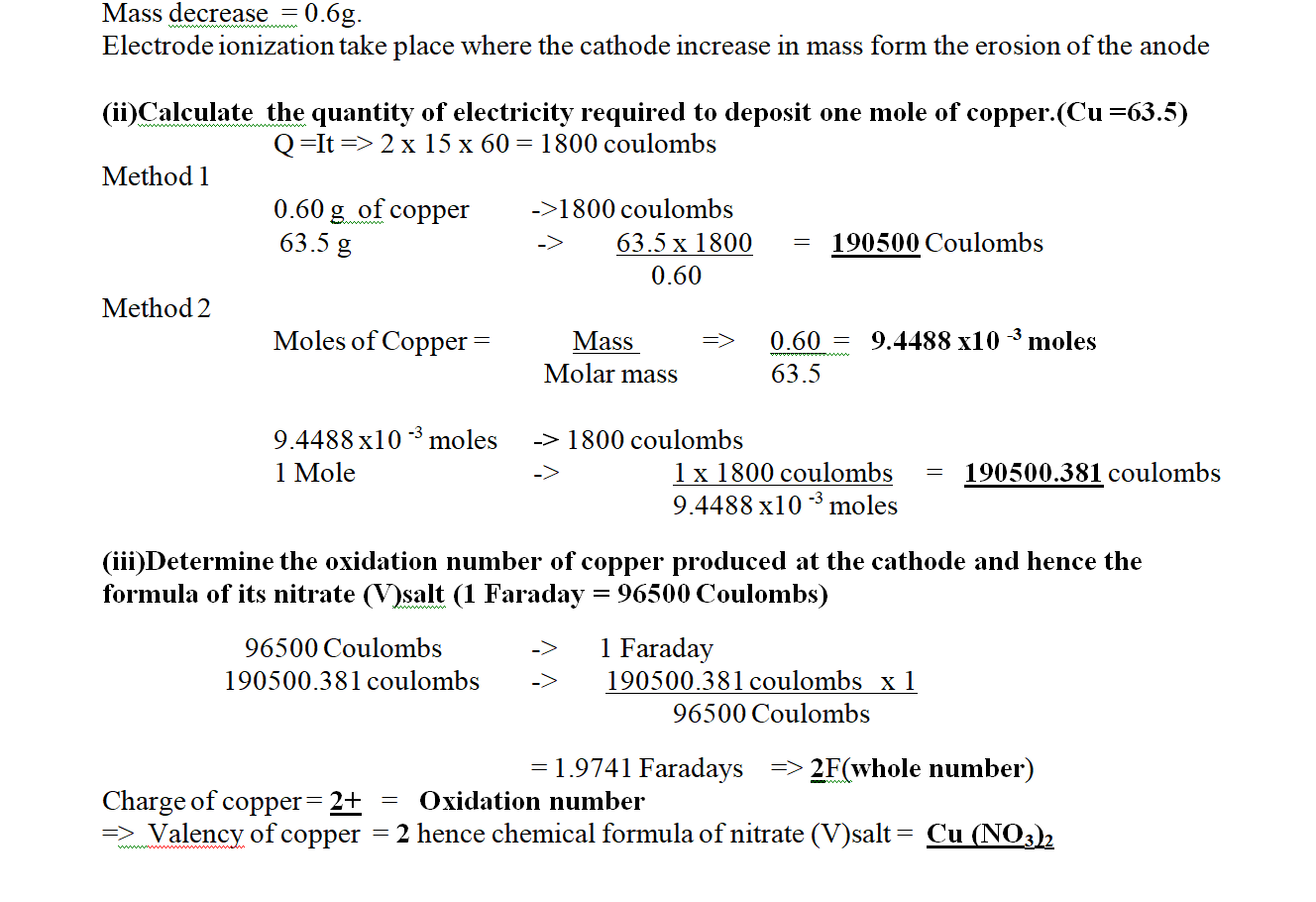
"Pdf" Revision Questions Chemistry Form 2
"Pdf" Revision Questions Chemistry Form 3
"Pdf" Revision Questions Chemistry Form 4
"Pdf" Revision Questions Chemistry Form Four
"Pdf" Revision Questions Chemistry Form One
"Pdf" Revision Questions Chemistry Form Three
"Pdf" Revision Questions Chemistry Form Two
1 a a KCSE Past Papers
10th Grade Chemistry Questions and Answers
10th Grade Chemistry Test
11th Ncert Chemistry
12th Class Chemistry Book Free Download
2014 KCSE Marking Schemes
2014 Pdf KCSE Past Papers 2015
2015 Chemistry Essay Questions and Answers Form 4
2016 KCSE Papers
2016 KCSE Prediction Questions
2017 Chemistry Hsc Answers
2017 KCSE Prediction Questions
2018 Chemistry KCSE Leakage
2018 Chemistry KCSE Questions
2018 KCSE Busineness Studies
2018 KCSE Exam
2018 KCSE Leakage
2018 KCSE Prediction Questions
2018 KCSE Questions
2019 Chemistry KCSE Leakage
2019 Chemistry KCSE Questions
2019 KCSE Leakage
2019 KCSE Questions
9th Grade Chemistry Study Guide
A a a Chemistry Notes
a a a Chemistry Notes!
a a a ChemistryNotes!
A a KCSE Past Papers
A Biblical View of Social Justice
A Level Chemistry Biological Molecules Questions
A Level Chemistry Exam Questions by Topic
A Level Chemistry Notes Edexcel
A Level Chemistry Notes Xtremepapers
A Level Chemistry Past Papers
A Level Chemistry Questions and Answers
a Level Chemistry Questions and Answers
A Level Chemistry Questions and Answers (Pdf)
A Level Chemistry Questions and Answers Pdf
A Level Chemistry Questions by Topic Kidney Questions With Markschemes
A Level Chemistry Revision
A Level Chemistry Revision Edexcel
A Level Chemistry Revision Guide
A Level Chemistry Revision Notes
A Level Chemistry Revision Notes Pdf
A Level Chemistry Textbook Pdf
A Level Chemistry Year 1 / as Aqa Exam Questions by Topic
A Level Edexcel Notes a* Chemistry
aa Chemistry Form 3 Questions and Answers
Advance KCSE Past Papers
Advance-africa.com KCSE Rev Quiz
Advantages and Disadvantages.
All Chemistry Essays
All Chemistry Notes for Senior Two
All KCSE Past Papers Chemistry With Making Schemes
All Marking Schemes Questions and Answers
All Past K.c.s.e Questions With Answers
Alliance Mocks 2017
Ap Bio Quizzes
Ap Chemistry 1 Textbook Pdf
Ap Chemistry Essay Questions and Answers
Are Sourced From KNEC.
As Level Chemistry Notes
Atika Chemistry Notes
Atika School Chemistry Notes
B/s Book 2 Notes
Basic Chemistry Books Pdf
basic Chemistry Interview Questions and Answers Pdf
Basic Chemistry Interview Questions and Answers Pdf
Basic Chemistry Pdf
Basic Chemistry Questions and Answers
Basic Chemistry Questions and Answers Pdf
Bbc Bitesize Chemistry Ks3
Bihar Board Chemistry Objective Answer 2017
Bihar Board Chemistry Objective Answer 2018
Bio Answers
Bio Quesions
Chemistry 0478
Chemistry 101
Chemistry 12th
Chemistry 12th Class Notes Pdf
Chemistry 2019 Syllabus
Chemistry All KCSE Short Notes
Chemistry Answers
Chemistry Answers Online Free
Chemistry Answers Quizlet
Chemistry Bk 2 Notes
Chemistry Book 1
Chemistry Book 1 Notes
Chemistry Book 2
Chemistry Book 2 Notes
Chemistry Book 3
Chemistry Book 3 KLB
Chemistry Book 3 Notes
Chemistry Book 4
Chemistry Book 4 Notes
Chemistry Book 4 Pdf
Chemistry Book for Class 11
Chemistry Book Four
Chemistry Book Four Notes
Chemistry Book One
Chemistry Book One Notes
Chemistry Book Pdf Free Download
Chemistry Book Three
Chemistry Book Three Notes
Chemistry Book Three Pdf
Chemistry Book Two
Chemistry Book Two Notes
Chemistry Books Form Three
Chemistry Bowl Chemistry Study Guide
Chemistry Bowl Questions Chemistry
Chemistry Bowl Questions Earth Chemistry
Chemistry Bowl Questions Math
Chemistry Bowl Questions Middle School
Chemistry Brekthrough Form Two Notes
Chemistry Class 12 Ncert Solutions
Chemistry Class 12 Pdf
Chemistry Communication Syllabus
Chemistry Diagram Software
Chemistry Diagrams for Class 11
Chemistry Diagrams for Class 12
Chemistry Diagrams for Class 9
Chemistry Diagrams for Class-10
Chemistry Diagrams in Form 1
Chemistry Diagrams in Form 2
Chemistry Diagrams in Form 3
Chemistry Diagrams in Form 4
Chemistry Diagrams Pdf
Chemistry Diagrams to Label
Chemistry Essay Questions and Answers
Chemistry Essay Questions and Answers 2018
Chemistry Essay Questions and Answers Form 1
Chemistry Essay Questions and Answers Form 2
Chemistry Essay Questions and Answers Form 3
Chemistry Essay Questions and Answers Form 4
Chemistry Essay Questions and Answers Form 4 Pdf
Chemistry Essay Questions and Answers Pdf
Chemistry Essay Revision Q
Chemistry Essays and Answers
Chemistry Essays Form One to Form Four
Chemistry Essays Form One to Form Three
Chemistry Essays KCSE
Chemistry Essays Pdf
Chemistry Exam 1 Multiple Choice
Chemistry Exam 2 Advance
Chemistry Exam 2 Test
Chemistry Exam 2016
Chemistry Exam Form Four
Chemistry Exam Form One
Chemistry Exam Form Three
Chemistry Exam Form Two
Chemistry Exam Practice Test
Chemistry Exam Questions
Chemistry Exam Questions and Answers
Chemistry Exam Questions and Answers Pdf
Chemistry Exam Study Guide
Chemistry Exams
Chemistry Excretion Notes
Chemistry Exercise Form 4 With Answers
Chemistry Final Exam Answer Key
Chemistry Final Exam Answer Key 2016
Chemistry Final Exam Answer Key 2017
Chemistry Final Exam Answers 2018
Chemistry Final Exam Answers 2019
Chemistry Final Exam Questions and Answers
Chemistry Fom 1 Notes
Chemistry Fom 2 Notes
Chemistry Fom 3 Notes
Chemistry Fom 4 Notes
Chemistry Form 1
Chemistry Form 1 & 2 and Answers
Chemistry Form 1 and 2 Essays
Chemistry Form 1 and 2 Essays Questions and Answers
Chemistry Form 1 Chapter 1
Chemistry Form 1 Diagrams
Chemistry Form 1 Exams
Chemistry Form 1 Mid Year Exam
Chemistry Form 1 Notes
Chemistry Form 1 Notes and Questions
Chemistry Form 1 Notes Download
Chemistry Form 1 Notes Free Download
Chemistry Form 1 Notes GCSE
Chemistry Form 1 Notes KCSE-kcse
Chemistry Form 1 Notes Pdf
Chemistry Form 1 Notes Pdf Download
Chemistry Form 1 Past Papers
Chemistry Form 1 Pdf
Chemistry Form 1 Pressure
Chemistry Form 1 Question Papers
Chemistry Form 1 Questions
Chemistry Form 1 Questions and Answers
Chemistry Form 1 Questions and Answers Pdf
Chemistry Form 1 Quiz
Chemistry Form 1 Revision Questions
Chemistry Form 1 Summary Notes
Chemistry Form 1 Syllabus
Chemistry Form 1 Work
Chemistry Form 1-4 Notes
Chemistry Form 2
Chemistry Form 2 Chapter 1
Chemistry Form 2 Chapter 2
Chemistry Form 2 Diagrams
Chemistry Form 2 Exam Paper 2014
Chemistry Form 2 Exams
Chemistry Form 2 Notes
Chemistry Form 2 Notes and Questions
Chemistry Form 2 Notes GCSE
Chemistry Form 2 Notes KCSE-kcse
Chemistry Form 2 Notes Pdf
Chemistry Form 2 Notes Pdf Download
Chemistry Form 2 Past Papers
Chemistry Form 2 Pdf
Chemistry Form 2 Question Papers
Chemistry Form 2 Questions
Chemistry Form 2 Questions and Answers
Chemistry Form 2 Questions and Answers Pdf
Chemistry Form 2 Quiz
Chemistry Form 2 Revision Notes
Chemistry Form 2 Salts
Chemistry Form 2 Structure and Bonding
Chemistry Form 2 Summary Notes
Chemistry Form 2 Syllabus
Chemistry Form 2 Work
Chemistry Form 3
Chemistry Form 3 and 4 Essays
Chemistry Form 3 and 4 Essays Questions and Answers
Chemistry Form 3 Chapter 3
Chemistry Form 3 Classification
Chemistry Form 3 Diagrams
Chemistry Form 3 Ecology
Chemistry Form 3 Exams
Chemistry Form 3 Notes
Chemistry Form 3 Notes and Questions
Chemistry Form 3 Notes GCSE
Chemistry Form 3 Notes KCSE-kcse
Chemistry Form 3 Notes Pdf
Chemistry Form 3 Notes Pdf Download
Chemistry Form 3 Notes Topic 1
Chemistry Form 3 Past Papers
Chemistry Form 3 Pdf
Chemistry Form 3 Question Papers
Chemistry Form 3 Questions
Chemistry Form 3 Questions and Answers
Chemistry Form 3 Questions and Answers Pdf
Chemistry Form 3 Questions and Answers Term 3
Chemistry Form 3 Questions and Answers+pdf
Chemistry Form 3 Quiz
Chemistry Form 3 Revision Notes
Chemistry Form 3 Revision Questions
Chemistry Form 3 Summary Notes
Chemistry Form 3 Syllabus
Chemistry Form 3 Syllabus Pdf
Chemistry Form 3 Topics
Chemistry Form 3 Work
Chemistry Form 4
Chemistry Form 4 All Chapter
Chemistry Form 4 Chapter 1 Conversion of Units
Chemistry Form 4 Chapter 1 Exercise
Chemistry Form 4 Chapter 1 Exercise and Answers
Chemistry Form 4 Chapter 1 Exercise Pdf
Chemistry Form 4 Chapter 1 Mind Map
Chemistry Form 4 Chapter 2
Chemistry Form 4 Chapter 2 Exercise and Answers
Chemistry Form 4 Chapter 2 Exercise Pdf
Chemistry Form 4 Chapter 2 Experiment
Chemistry Form 4 Chapter 2 Formula
Chemistry Form 4 Chapter 2 Mind Map
Chemistry Form 4 Chapter 2 Momentum
Chemistry Form 4 Chapter 2 Notes Pdf
Chemistry Form 4 Chapter 2 Objective Questions and Answers
Chemistry Form 4 Chapter 2 Paper 2
Chemistry Form 4 Chapter 2 Slideshare
Chemistry Form 4 Chapter 3
Chemistry Form 4 Chapter 3 Questions and Answers
Chemistry Form 4 Chapter 4
Chemistry Form 4 Chapter 4 Notes Pdf
Chemistry Form 4 Chapter 5 Light Questions and Answers
Chemistry Form 4 Chapter 5 Notes Pdf
Chemistry Form 4 Diagrams
Chemistry Form 4 Exam Paper 1
Chemistry Form 4 Exams
Chemistry Form 4 Exercise
Chemistry Form 4 Exercise Pdf
Chemistry Form 4 Module With Answer
Chemistry Form 4 Note
Chemistry Form 4 Notes
Chemistry Form 4 Notes (Pdf)
Chemistry Form 4 Notes All Chapter Pdf
Chemistry Form 4 Notes and Questions
Chemistry Form 4 Notes Chapter 1
Chemistry Form 4 Notes Chapter 2
Chemistry Form 4 Notes Chapter 3
Chemistry Form 4 Notes Download
Chemistry Form 4 Notes Free Download
Chemistry Form 4 Notes GCSE
Chemistry Form 4 Notes KCSE-kcse
Chemistry Form 4 Notes Pdf
Chemistry Form 4 Notes Pdf Download
Chemistry Form 4 Paper 2 Questions and Answers
Chemistry Form 4 Past Papers
Chemistry Form 4 Question Papers
Chemistry Form 4 Questions
Chemistry Form 4 Questions and Answers
Chemistry Form 4 Questions and Answers Pdf
Chemistry Form 4 Quiz
Chemistry Form 4 Revision Notes
Chemistry Form 4 Schemes of Work
Chemistry Form 4 Summary Notes
Chemistry Form 4 Syllabus
Chemistry Form 4 Textbook Pdf
Chemistry Form 4 Work
Chemistry Form 5 Chapter 1 Exercise and Answers
Chemistry Form 5 Chapter 1 Notes Pdf
Chemistry Form 5 Chapter 2 Notes Pdf
Chemistry Form 5 Chapter 2 Slideshare
Chemistry Form 5 Chapter 3 Notes Pdf
Chemistry Form 5 Notes Pdf
Chemistry Form Four Book
Chemistry Form Four Notes
Chemistry Form Four Notes and Questions
Chemistry Form Four Notes GCSE
Chemistry Form Four Notes Pdf
Chemistry Form Four Past Papers
Chemistry Form Four Questions
Chemistry Form Four Questions and Answers
Chemistry Form Four Questions and Answers Pdf
Chemistry Form Four Quiz
Chemistry Form Four Study Notes
Chemistry Form Four Syllabus
Chemistry Form Four Topic 2
Chemistry Form Four Topic 4
Chemistry Form Four Topics
Chemistry Form Four Work
Chemistry Form One
Chemistry Form One Book
Chemistry Form One Book Pdf
Chemistry Form One Download Topic 1 Upto 3
Chemistry Form One Exam
Chemistry Form One Notes
Chemistry Form One Notes and Questions
Chemistry Form One Notes GCSE
Chemistry Form One Notes Pdf
Chemistry Form One Pdf
Chemistry Form One Questions
Chemistry Form One Questions and Answers
Chemistry Form One Questions and Answers Pdf
Chemistry Form One Questions and Their Answers
Chemistry Form One Quiz
Chemistry Form One Revision Question
Chemistry Form One Schemes of Work
Chemistry Form One Study Notes
Chemistry Form One Syllabus
Chemistry Form One Term Three Test
Chemistry Form One to Three Notes
Chemistry Form One Work
Chemistry Form Three
Chemistry Form Three Book
Chemistry Form Three Notes
Chemistry Form Three Notes and Questions
Chemistry Form Three Notes GCSE
Chemistry Form Three Questions and Answers
Chemistry Form Three Questions and Answers Pdf
Chemistry Form Three Quiz
Chemistry Form Three Reproduction
Chemistry Form Three Reproduction.
Chemistry Form Three Study Notes
Chemistry Form Three Work
Chemistry Form Three-questions and Answers
Chemistry Form Two
Chemistry Form Two Book
Chemistry Form Two Diagrams
Chemistry Form Two Notes
Chemistry Form Two Notes and Questions
Chemistry Form Two Notes GCSE
Chemistry Form Two Notes Pdf
Chemistry Form Two Notes-pdf
Chemistry Form Two Pdf
Chemistry Form Two Questions
Chemistry Form Two Questions and Answers
Chemistry Form Two Questions and Answers Pdf
Chemistry Form Two Quiz
Chemistry Form Two Study Notes
Chemistry Form Two Topics
Chemistry Form Two Work
Chemistry Form Two,schemes of Work
Chemistry Form2
Chemistry Form2 Textbook
Chemistry Game Form Four Question End Answers
Chemistry Grade 10 Exam Papers
Chemistry Hsc Pdf
Chemistry Human Reproduction Video
Chemistry IGCSE Past Papers Xtremepapers
Chemistry K.c.s.e 2017
Chemistry KCSE
Chemistry KCSE 2016
Chemistry KCSE 2017
Chemistry KCSE 2017 Paper 1
Chemistry KCSE Past Papers
Chemistry KCSE Questions
Chemistry KCSE Questions and Answer
Chemistry KCSE Quizzes & Answers
Chemistry KCSE Revision
Chemistry KCSE Revision Notes
Chemistry KCSE Setting Questions Form One and Two
Chemistry Ksce 2015
Chemistry Last Year K.c.s.e Questions
Chemistry Lesson Plan Form Two
Chemistry Made Familiar
Chemistry Mcq for Class 11
Chemistry Mcq for Class 12
Chemistry Mcq for Competitive Exams
Chemistry Mcq for Competitive Exams Pdf
Chemistry Mcq for Neet Pdf
Chemistry Mcq for Ssc
Chemistry Mcq Questions With Answers
Chemistry Mcq With Answers Pdf
Chemistry Mcqs for Class 12 Pdf
Chemistry Mcqs With Answers Pdf
Chemistry Mid Familia Form One
Chemistry Mock Papers
Chemistry Module Form 5
Chemistry Multiple Choice Questions and Answers Cxc
Chemistry Multiple Choice Questions and Answers Pdf
Chemistry Multiple Choice Questions With Answers Pdf
Chemistry Note
Chemistry Note Form Two All Chapters
Chemistry Notes
Chemistry Notes and Guestion and Answear
Chemistry Notes and Syllabus
Chemistry Notes Class 10
Chemistry Notes for Class 11 Pdf
Chemistry Notes for Class 12 Pdf
Chemistry Notes for High School Students
Chemistry Notes for IGCSE 2014
Chemistry Notes Form 1
Chemistry Notes Form 1 4
Chemistry Notes Form 1 Free Download
Chemistry Notes Form 1 KLB
Chemistry Notes Form 1 Pdf
Chemistry Notes Form 1-4
Chemistry Notes Form 1-4(1) Chemistry
Chemistry Notes Form 14
Chemistry Notes Form 2
Chemistry Notes Form 2 KLB
Chemistry Notes Form 2 Pdf
Chemistry Notes Form 2; Chemistry Notes
Chemistry Notes Form 3
Chemistry Notes Form 3 KLB
Chemistry Notes Form 3 Pdf
Chemistry Notes Form 4
Chemistry Notes Form 4 Chapter 2
Chemistry Notes Form 4 KLB
Chemistry Notes Form 4 Pdf
Chemistry Notes Form 4-pdf
Chemistry Notes Form Four
Chemistry Notes Form Four KLB
Chemistry Notes Form Four Pdf
Chemistry Notes Form One
Chemistry Notes Form One KLB
Chemistry Notes Form One Pdf
Chemistry Notes Form One to Form Four
Chemistry Notes Form Three
Chemistry Notes Form Three KLB
Chemistry Notes Form Three Pdf
Chemistry Notes Form Two
Chemistry Notes Form Two KLB
Chemistry Notes Form Two Pdf
Chemistry Notes Form2
Chemistry Notes IGCSE
Chemistry Notes Kenya
Chemistry Notes on Agroforestry
Chemistry Notes Pdf
Chemistry Notes:
Chemistry Objective Answer
Chemistry Objective Answer 2018
Chemistry Objective Questions for Competitive Exams
Chemistry Objective Questions for Competitive Exams Pdf
Chemistry Oral Exam Questions
Chemistry Paper 1
Chemistry Paper 1 2018 Marking Rules
Chemistry Paper 1 Notes
Chemistry Paper 1 Questions
Chemistry Paper 1 Questions and Answers
Chemistry Paper 1 Topics
Chemistry Paper 1 With Answers
Chemistry Paper 2
Chemistry Paper 2 2017
Chemistry Paper 2 2018 Marking Rules
Chemistry Paper 2 Questions and Answers
Chemistry Paper 2 Questions and Answers Pdf
Chemistry Paper 2 Revision
Chemistry Paper 2 Topics
Chemistry Paper 2018
Chemistry Paper 3 2018 Marking Rules
Chemistry Paper 3 Question and Answer
Chemistry Paper 3 Question Paper 2014 KCSE
Chemistry Paper 3 Question Paper 2015 KCSE
Chemistry Paper 3 Question Paper 2016 KCSE
Chemistry Paper 3 Question Paper 2017 KCSE
Chemistry Paper 3 Question Paper 2018 KCSE
Chemistry Paper 3 Questions and Answers
Chemistry Paper One Questions and Answers
Chemistry Paper One Topics
Chemistry Paper Two Qestions With Answers
Chemistry Paper1
Chemistry Paper2
Chemistry Paper3
Chemistry Paper4
Chemistry Past Papers
Chemistry Past Papers 2017
Chemistry Past Papers a Level
Chemistry Past Papers Form 1
Chemistry Past Papers Form 2
Chemistry Past Papers Form 3
Chemistry Past Papers O Level
Chemistry Pdf Download
Chemistry Pp1 KCSE 2016
Chemistry Practical Book Class 12 Pdf
Chemistry Practical Exam
Chemistry Practicals Form One
Chemistry Practicals Questions and Answers
Chemistry Practice Test 9th Grade
Chemistry Practice Test Answers
Chemistry Practice Test Questions and Answers
Chemistry Practice Test Quizlet
Chemistry Predicted Questions This Year KCSE
Chemistry Preparation Notes
Chemistry Pretest High School Pdf
Chemistry Question and Answer With Explanation
Chemistry Question and Answers 2019
Chemistry Question and Answers 2020
Chemistry Question and Answers 2021
Chemistry Question and Answers 2022
Chemistry Question and Answers Note
Chemistry Questions
Chemistry Questions and Answers
Chemistry Questions and Answers for High School
Chemistry Questions and Answers for High Schools
Chemistry Questions and Answers for High Schools Pdf
Chemistry Questions and Answers for Secondary Schools
Chemistry Questions and Answers Form 1
Chemistry Questions and Answers Form 2
Chemistry Questions and Answers Form 3
Chemistry Questions and Answers Form 4
Chemistry Questions and Answers Multiple Choice
Chemistry Questions and Answers Notes
Chemistry Questions and Answers O
Chemistry Questions and Answers Online
Chemistry Questions and Answers Pdf
Chemistry Questions and Answers Pdf for Class 12
Chemistry Questions and Answers Pdf for Competitive Exams
Chemistry Questions and Answers-form 2
Chemistry Questions for High School
Chemistry Questions for High School Students With Answers
Chemistry Questions for Senior 1
Chemistry Questions for Senior 2
Chemistry Questions for Senior 3
Chemistry Questions for Senior 4
Chemistry Questions for Senior 5
Chemistry Questions for Senior 6
Chemistry Questions for Senior Five
Chemistry Questions for Senior Four
Chemistry Questions for Senior One
Chemistry Questions for Senior Six
Chemistry Questions for Senior Three
Chemistry Questions for Senior Two
Chemistry Questions Form One
Chemistry Questions Multiple Choice
Chemistry Questions Quizlet
Chemistry Questions to Ask Your Teacher
Chemistry Quetion and Answer Form Four
Chemistry Quetion and Answer Form One
Chemistry Quetion and Answer Form Three
Chemistry Quetion and Answer Form Two
Chemistry Quiz for Class 9
Chemistry Quiz for Class 9 Chemistry
Chemistry Quiz Questions and Answers for Class 10
Chemistry Quiz Questions and Answers for Class 10 Pdf
Chemistry Quiz Questions and Answers for Class 12
Chemistry Quiz Questions and Answers for Class 9
Chemistry Quiz Questions and Answers for Class 9 Pdf
Chemistry Quiz Questions and Answers for High School
Chemistry Quiz Questions and Answers Multiple Choice
Chemistry Quiz Questions and Answers Pdf
Chemistry Quiz Questions for Class 12
Chemistry Quiz Questions for College Students
Chemistry Quiz With Answers
Chemistry Quiz With Answers Pdf
Chemistry Quizlet
Chemistry Revision
Chemistry Revision a Level
Chemistry Revision Chemistry Notes Chemistry
Chemistry Revision Exam
Chemistry Revision Examination
Chemistry Revision Form One
Chemistry Revision Notes
Chemistry Revision Notes Chemistry
Chemistry Revision Notes Form 1
Chemistry Revision Notes Form 2
Chemistry Revision Notes Form 3
Chemistry Revision Notes Form 4
Chemistry Revision Notes IGCSE
Chemistry Revision Paper One
Chemistry Revision Questions
Chemistry Revision Questions and Answers
Chemistry Revision Questions and Answers Form 1
Chemistry Revision Questions and Answers Form 2
Chemistry Revision Questions and Answers Form 3
Chemistry Revision Questions and Answers Form 4
Chemistry Revision Questions and Answers Form Four
Chemistry Revision Questions and Answers Form One
Chemistry Revision Questions and Answers Form Three
Chemistry Revision Questions and Answers Form Two
Chemistry Revision Questions Form 1
Chemistry Revision Questions Form 2
Chemistry Revision Questions Form 3
Chemistry Revision Questions Form 4
Chemistry Revision Questions Form Four
Chemistry Revision Questions Form One
Chemistry Revision Questions Form Three
Chemistry Revision Questions Form Two
Chemistry Revision Quiz
Chemistry Revision Test
Chemistry Secondary School Revision
Chemistry Simple Notes
Chemistry Spm Notes Download
Chemistry Spm Notes Pdf
Chemistry Spm Questions
Chemistry Study Form 2
Chemistry Study Guide
Chemistry Study Guide Answer Key
Chemistry Study Guide Answers
Chemistry Study Guide Chemistry Questions and Answers
Chemistry Study Guide Ib
Chemistry Study Guide Pdf
Chemistry Study Guides
Chemistry Study Notes
Chemistry Study Notes Materials Form 1 Pdf
Chemistry Study Notes Materials Form 2 3 Pdf
Chemistry Study Notes Materials Form 2 Pdf
Chemistry Study Notes Materials Form 3 Pdf
Chemistry Study Notes Materials Form 4 Pdf
Chemistry Syllabus in Kenya
Chemistry Syllabus Pdf
Chemistry Test 1 Quizlet
Chemistry Test Questions
Chemistry Test Questions and Answers
Chemistry Test Questions and Answers Pdf
Chemistry Topic One Form Four
Chemistry Topics Form One
Chemistry Unit 1 Quiz
Chemistry Vol 3
Chemistry | Revision Chemistry
Chemistry,form 4
Chemistry.form Four.topic Three
ChemistryExam Form Three
ChemistryModule Form 5
ChemistryNotes
ChemistryNotes for Class 11 Pdf
ChemistryNotes for Class 12 Pdf
ChemistryNotes Form 1
ChemistryNotes Form 1 Free Download
ChemistryNotes Form 2
ChemistryNotes Form 3
ChemistryNotes Form 3 Pdf
ChemistryNotes IGCSE
ChemistryNotes Pdf
ChemistryPast Papers
ChemistryQuestions and Answers Pdf
ChemistrySimple Notes
ChemistrySpm Notes Download
ChemistrySpm Notes Pdf
ChemistrySpm Questions
ChemistryStudy Guide Answers
ChemistryStudy Guide Pdf
ChemistryStudy Guides
Blologytextpapers
Bridge Chemistry
Business Past KCSE Past Papers
Business Studies Form 3 Notes Pdf
Business Studies Form 4 Notes Pdf
C R E Form One KLB
C R E Form One Oli Topic
C.r.e Form 1 Notes Kenya
C.r.e Form 2 Notes Kenya
C.r.e Form 3 Notes
C.r.e Form 3 Notes Kenya
C.r.e Form 3 Pdf
C.r.e Form 4 Notes Kenya
C.r.e Form One Notes Pdf
C.r.e Notes Form 1
C.r.e Revision Notes
C.r.e Short Notes
Cambridge IGCSE Chemistry
Cambridge IGCSE Chemistry 3rd Edition
Cambridge IGCSE Chemistry 3rd Edition Plus Cd South Asia Edition
Cambridge IGCSE Chemistry Answers
Cambridge IGCSE Chemistry Coursebook Pdf Download
Cambridge IGCSE Chemistry Practical Workbook
Cambridge IGCSE Chemistry Revision Guide Pdf
Cambridge IGCSE Chemistry Study and Revision Guide 2nd Edition Pdf
Cambridge IGCSE Chemistry Study and Revision Guide Pdf
Cambridge IGCSE Chemistry Workbook Free Download
Cambridge IGCSE Chemistry Workbook Pdf
Cambridge IGCSE® Chemistry Coursebook
Caucasian Chalk Circle Essay Questions
Chapter 1 Introduction to Chemistry
Chapter 1 Introduction to Chemistry Studies
Cie a Level Chemistry Notes 2016
Cie a Level Chemistry Notes Pdf
Cie Past Papers
Class 10 Chemistry Chapter 1 Mcqs
Class 8 Chemistry Notes KCSE-kcse
College Chemistry Notes
College Chemistry Practice Test
College Chemistry Quiz
College Chemistry Quiz Chapter 1
College Chemistry Quizlet
College Chemistry Study Guide
College Chemistry Study Guide Pdf
College Chemistry Test Questions and Answers
College Chemistry Volume 3 Pdf
College ChemistryNotes
Complete Chemistry for Cambridge IGCSE
Complete Chemistry for Cambridge IGCSE Revision Guide Pdf
County Mocks 2017
Cse Past Papers Chemistry 2017
Dl Chemistry Form 3 Pdf Kusoma
Download Chemistry Form 1
Download Chemistry Form 2
Download Chemistry Form 2 Notes
Download Chemistry Form 3
Download Chemistry Form 3 Notes
Download Chemistry Form 4
Download Chemistry Form Four
Download Chemistry Form One
Download Chemistry Form Three
Download Chemistry Form Two
Download Chemistry Notes Form 3
Download Chemistry Notes Form One
Download ChemistryNotes Form 3
Download Form Three Chemistry Notes
Download Free KCSE Past Papers Chemistry
Download Free KCSE Past Papers From KNEC.
Download KCSE Past Papers With Answers
Download KCSE Revision Notes
Download KLB Chemistry Book 2
Download KLB Chemistry Book 3
Download KLB Chemistry Book 4
Download Notes of Chemistry
Downloads | Chemistry | Form Four Exams | Exams
Downloads | Chemistry | Form One Exams | Exams
Downloads | Chemistry | Form Three Exams | Exams
Downloads | Chemistry | Form Two Exams | Exams
Downloads | KCSE Papers and Marking Schemes |
Dvance KCSE Past Papers
Easy Chemistry Questions
Edexcel a Level Chemistry B
Edexcel a Level Chemistry Notes Pdf
Edexcel a Level Chemistry Salters Nuffield
Edexcel A2 Chemistry Notes
Edexcel as Chemistry Revision Guide Pdf
Edexcel Chemistry A2 Revision Notes Pdf
Edexcel Chemistry Unit 2 Revision Notes
Edexcel GCSE Chemistry Revision Guide Pdf
Edexcel IGCSE Chemistry Past Papers
Edexcel IGCSE Chemistry Revision Guide Free Pdf Download
Edexcel IGCSE Chemistry Revision Guide Pdf
Edexcel IGCSE Chemistry Revision Guide Pdf Download
Electronics Form Four Notes
Energy Questions Chemistry Bowl
Essay Questions and Answers KCSE Chemistry Notes
Essay Questions and Answers on Betrayal in the City
Essay Questions Based on Betrayal in the City
Evolving World Chemistry Book 1 Pdf
Evolving World Chemistry Book 4 Notes
Evolving World Chemistry Book Form 1
Evolving World-history Book 3
Exam Notes for Chemistry 101
Exams KCSE Chemistry Paper 1 Questions and Answers
F3 Chemistry Test Paper
Find Download KCSE Past Papers With Answers
Find KCSE Chemistry Essay Questions and Answers
Form 1 Chemistry Exam
Form 1 Chemistry Notes
Form 1 Chemistry Questions and Answers
Form 1 Chemistry Questions and Answers Pdf
Form 1 Chemistry Revision Notes
Form 1 Chemistry Summurized Revision Pdf
Form 1 Chemistry Syllabus
Form 1 Chemistry Test Paper Pdf
Form 1 Chemistry Topics
Form 1 ChemistryNotes
Form 1 ChemistryQuestions and Answers
Form 1 ChemistryRevision Notes
Form 1 ChemistrySyllabus
Form 1 ChemistryTest Paper Pdf
Form 1 Past Papers
Form 1 Past Papers With Answers
Form 1 Revision Papers
Form 1 Subjects in Kenya
Form 2 Chemistry Exam
Form 2 Chemistry Exam Paper
Form 2 Chemistry Exam Paper 2016
Form 2 Chemistry Exam Paper Free Download
Form 2 Chemistry Exam Paper With Answer
Form 2 Chemistry Final Year Exam Paper 2
Form 2 Chemistry Notes
Form 2 Chemistry Notes and Revision Questions
Form 2 Chemistry Notes Pdf
Form 2 Chemistry Past Papers
Form 2 Chemistry Questions
Form 2 Chemistry Questions and Answers
Form 2 Chemistry Questions and Answers >
Form 2 Chemistry Questions and Answers Pdf
Form 2 Chemistry Revision Notes
Form 2 Chemistry Short Notes
Form 2 Chemistry Syllabus
Form 2 ChemistryExam Paper
Form 2 ChemistryExam Paper Free Download
Form 2 ChemistryExam Paper With Answer
Form 2 ChemistryFinal Year Exam Paper 2
Form 2 ChemistryPast Papers
Form 2 ChemistryRevision Notes
Form 2 ChemistryShort Notes
Form 2 ChemistrySyllabus
Form 2 Revision Papers
Form 2 Subjects in Kenya
Form 3 Chemistry Book
Form 3 Chemistry Exam
Form 3 Chemistry Exam Paper
Form 3 Chemistry Notes
Form 3 Chemistry Past Papers
Form 3 Chemistry Questions
Form 3 Chemistry Questions and Answers
Form 3 Chemistry Questions and Answers Pdf
Form 3 Chemistry Revision Notes
Form 3 Chemistry Syllabus
Form 3 ChemistryExam Paper
Form 3 ChemistryNotes
Form 3 ChemistryPast Papers
Form 3 ChemistryQuestions
Form 3 ChemistryQuestions and Answers Pdf
Form 3 ChemistryRevision Notes
Form 3 ChemistrySyllabus
Form 3 C.r.e
Form 3 Notes of Chemistry Topic on Fish
Form 3 Past Papers
Form 3 Revision Papers
Form 3 Subjects in Kenya
Form 4 Chemistry Exam
Form 4 Chemistry Notes
Form 4 Chemistry Notes Pdf
Form 4 Chemistry Questions and Answers
Form 4 Chemistry Questions and Answers Pdf
Form 4 Chemistry Revision Notes
Form 4 Chemistry Syllabus
Form 4 Chemistry Topics
Form 4 ChemistryNotes
Form 4 ChemistryRevision Notes
Form 4 ChemistrySyllabus
Form 4 ChemistryTopics
Form 4 Exam Papers
Form 4 Revision Papers
Form 4 Subjects in Kenya
Form 5 Chemistry Topics
Form 5 ChemistryTopics
Form Five Chemistry Notes
Form Five ChemistryNotes
Form Four Chemistry Book
Form Four Chemistry Notes
Form Four Chemistry Notes Pdf
Form Four Chemistry Questions and Answers
Form Four Chemistry Questions and Answers Pdf
Form Four Chemistry Revision Questions
Form Four Chemistry Syllabus
Form Four Chemistry Topics
Form Four ChemistryNotes
Form Four ChemistryQuestions and Answers
Form Four ChemistryQuestions and Answers Pdf
Form Four ChemistryTopics
Form Four Notes
Form Four Revision Papers
Form Four Subjects in Kenya
Form One Chemistry Book
Form One Chemistry Examination
Form One Chemistry First Topic
Form One Chemistry Lesson Plan
Form One Chemistry Notes Pdf
Form One Chemistry Past Papers Pdf
Form One Chemistry Questions
Form One Chemistry Questions and Answers
Form One Chemistry Questions and Answers Pdf
Form One Chemistry Revision Questions
Form One Chemistry Short Notes
Form One Chemistry Syllabus
Form One Chemistry Topics
Form One ChemistryExamination
Form One ChemistryPast Papers Pdf
Form One ChemistryQuestions and Answers
Form One ChemistryQuestions and Answers Pdf
Form One ChemistryTopics
Form One Exams
Form One Notes of Chemistry
Form One Past Papers
Form One Subjects in Kenya
Form One Term One Chemistry Exam
Form One Term One ChemistryExam
Form Three Chemistry Book
Form Three Chemistry Book Pdf
Form Three Chemistry Notes
Form Three Chemistry Notes Pdf
Form Three Chemistry Questions and Answers
Form Three Chemistry Questions and Answers Pdf
Form Three Chemistry Revision Questions
Form Three Chemistry Syllabus
Form Three Chemistry Topics
Form Three ChemistryNotes
Form Three ChemistryNotes Pdf
Form Three ChemistryQuestions and Answers
Form Three ChemistryQuestions and Answers Pdf
Form Three ChemistryTopics
Form Three Subjects in Kenya
Form Two Chemistry Book
Form Two Chemistry Cat
Form Two Chemistry Examination
Form Two Chemistry Notes
Form Two Chemistry Notes Pdf
Form Two Chemistry Past Papers
Form Two Chemistry Questions and Answers
Form Two Chemistry Questions and Answers Pdf
Form Two Chemistry Revision Questions
Form Two Chemistry Syllabus
Form Two Chemistry Topics
Form Two ChemistryNotes
Form Two ChemistryNotes Pdf
Form Two ChemistryQuestions and Answers
Form Two ChemistryQuestions and Answers Pdf
Form Two ChemistrySyllabus
Form Two ChemistryTopics
Form Two Notes
Form Two Subjects in Kenya
Free a-level Chemistry Revision App | Pass Your Chemistry Exams
Free Chemistry Form 1 Notes
Free Chemistry Notes Form 1
Free Chemistry Notes Pdf
Free ChemistryNotes Pdf
Free College Chemistry Practice Test
Free Form1,form2,form3 Past Papers Free KCSE Past Papers
Free KCSE Mocks 2015
Free KCSE Past Papers 2014
Free KCSE Past Papers KCSE Past
Free KCSE Past Papers Kenya,
Free KCSE Past Papers With Answers
Free KCSE Questions and Answers on Chemistry
Free KCSE Revision Notes
Free Marking Schemes
Free Mocks Online KCSE Answers Past Exams Question Papers
Free Revision Papers
From Three Notes Topic One KLB
Fun Chemistry Questions
Funny Chemistry Questions
Funny Chemistry Questions and Answers
Funny Chemistry Questions to Ask
Funny Chemistry Quotes
GCSE Chemistry Exam Questions and Answers
GCSE Chemistry Past Papers
GCSE Chemistry Revision
GCSE Chemistry Revision Notes
GCSE Chemistry Revision Notes Pdf
GCSE Chemistry Revision Notes Pdf 9-1
GCSE Chemistry Revision Questions and Answers
GCSE Chemistry Textbook Pdf
GCSE Chemistry Topics Pass My Exams: Easy Exam Revision Notes
General Chemistry Notes Pdf
General Chemistry Practice Test With Answers
General Chemistry Quiz
General Chemistry Quiz Pdf
General Chemistry Test Questions and Answers
General Chemistry Test Questions and Answers Pdf
General Knowledge in Chemistry Human Body
Good Chemistry Questions to Ask
GRE Chemistry Practice Test
GRE Chemistry Subject Test Pdf
Handbook of Chemistry Pdf Free Download
Hard Chemistry Questions
Hard Chemistry Questions and Answers
Hard Chemistry Questions to Ask Your Teacher
Hard Chemistry Quiz Questions
Hard Form 3 Chemistry Question
High School Chemistry Final Exam Doc
High School Chemistry Final Exam Pdf
High School Chemistry Final Exam Questions
High School Chemistry Final Exam Questions and Answers
High School Chemistry Notes
High School Chemistry Practice Test
High School Chemistry Pretest With Answers
High School Chemistry Questions and Answers Pdf
High School Chemistry Study Guide
High School Chemistry Test Questions and Answers Pdf
High School ChemistryNotes
High School ChemistryStudy Guide
How to Answer KCSE Chemistry Question
How to Motivate a Form 4 Student
How to Motivate a KCSE Candidate
How to Motivate a KCSE Student
How to Pass Chemistry Questions & Answers Form 1&2 | Text Book
How to Revise Chemistry
How to Revise Effectively for KCSE
How to Study Chemistry: 5 Study Techniques to Master Chemistry
Hsc Chemistry 2018
Hsc Chemistry 2019
Https://www.knec.ac.ke/ Www.knec-portal.ac.ke/ KNEC Portal:
Ial Chemistry Notes
Ib Chemistry Cold War Notes
Ib Chemistry Notes
Ib Chemistry Notes Pdf
Ib Chemistry of the Americas Notes
Ib Chemistry of the Americas Study Guide
Ib Chemistry Paper 2 Study Guide
Ib Chemistry Question Bank by Topic
Ib Chemistry Study Guide Pdf
Ict Notes Form 1
IGCSE Chemistry Alternative to Practical Revision
IGCSE Chemistry Alternative to Practical Revision Notes
IGCSE Chemistry Book
IGCSE Chemistry Book Pdf Download
IGCSE Chemistry Notes
IGCSE Chemistry Notes 2017 Pdf
IGCSE Chemistry Notes Edexcel
IGCSE Chemistry Paper 2 Notes
IGCSE Chemistry Paper 6 Notes
IGCSE Chemistry Past Papers
IGCSE Chemistry Past Papers 2014
IGCSE Chemistry Past Papers 2017
IGCSE Chemistry Pdf
IGCSE Chemistry Pre Release Material 2018
IGCSE Chemistry Resources
IGCSE Chemistry Revision Guide
IGCSE Chemistry Revision Guide Free Download
IGCSE Chemistry Revision Guide Pdf Download
IGCSE Chemistry Revision Notes Pdf
IGCSE Chemistry Revision Worksheets
IGCSE Chemistry Workbook Pdf
IGCSE Chemistry Znotes
IGCSE ChemistryPast Papers
IGCSE Notes Chemistry
Importance of Agroforestry
Inorganic Chemistry Multiple Choice Questions With Answers Pdf
Inorganic Chemistry Questions and Answers Pdf
Interesting Chemistry Questions
Interesting Chemistry Questions and Answers
Interesting Questions to Ask About Chemistry
Intro to Chemistry Quiz
Introduction of Chemistry Form One
Introduction to Chemistry
Introduction to Chemistry Notes
Introduction to Chemistry Pdf
Introduction to ChemistryNotes
Is Agroforestry Sustainable?
K.c.s.e Answers Chemistry Paper One 2018
K.c.s.e Chemistry 2017
K.c.s.e Chemistry 2018
K.c.s.e Chemistry Paper 1 2017
K.c.s.e Mocks 2018
K.c.s.e Papers 2015
K.c.s.e Papers 2016
K.c.s.e Past Papers 2014
K.c.s.e.Chemistry Paper 2 Year 2018
K.c.s.e.results 2018 for Busia County
K.l.b Chemistry Form 3
K.l.b Chemistry Notes
K.l.b ChemistryNotes
Kasneb Past Papers for Colleges Chemistry Past Papers
KCSE 2010 Marking Scheme
KCSE 2010 Past Papers
KCSE 2011 Chemistry Paper 1
KCSE 2011 Marking Scheme
KCSE 2012 Chemistry Paper 2 Marking Scheme
KCSE 2012 Marking Schemes
KCSE 2013 Chemistry Paper 1
KCSE 2013 Marking Scheme
KCSE 2013 Marking Scheme Pdf
KCSE 2014
KCSE 2015 Chemistry Paper 2
KCSE 2015 Chemistry Paper 3
KCSE 2015 Marking Scheme
KCSE 2015 Past Papers
KCSE 2016 Chemistry Paper 1
KCSE 2016 Chemistry Paper 2
KCSE 2017 Chemistry Paper 1
KCSE 2017 Chemistry Paper 2
KCSE 2017 Hostory Papers With Answers.com
KCSE 2017 Marking Scheme
KCSE 2017 Papers
KCSE 2017 Papers and Marking Scheme
KCSE 2017 Papers Pdf
KCSE 2017 Past Papers
KCSE 2017 Prediction Pdf
KCSE 2018 Chemistry and Answers
KCSE 2018 Chemistry Prediction
KCSE 2018 Leakage
KCSE 2018 Marking Scheme
KCSE 2018 Papers
KCSE 2018 Prediction Pdf
KCSE 2018 Predictions
KCSE 2018 Questions
KCSE 2018 Questions and Answers
KCSE 2019 Leakage Chemistry
KCSE 2019 Marking Scheme
KCSE 2019 Questions
KCSE 2019 Questions and Answers
KCSE 2020 Questions
KCSE 2020 Questions and Answers
KCSE Answers
KCSE Answers Past Exams Question Papers Downloads |
KCSE Chemistry 2011
KCSE Chemistry 2016
KCSE Chemistry Diagramsbiology Revision Tips
KCSE Chemistry Essay Questions and Answers
KCSE Chemistry Essay Questions and Answers Pdf
KCSE Chemistry Essays
KCSE Chemistry Essays Pdf
KCSE Chemistry Marking Schemes
KCSE Chemistry Notes
KCSE Chemistry Notes Pdf
KCSE Chemistry Notes, Syllabus, Questions, Answers
KCSE Chemistry Paper 1
KCSE Chemistry Paper 1 2011
KCSE Chemistry Paper 1 2012
KCSE Chemistry Paper 1 2013
KCSE Chemistry Paper 1 2015
KCSE Chemistry Paper 1 2016
KCSE Chemistry Paper 1 2017
KCSE Chemistry Paper 1 2017 Pdf
KCSE Chemistry Paper 1 Questions and Answers
KCSE Chemistry Paper 2
KCSE Chemistry Paper 2 2012
KCSE Chemistry Paper 2 2012 KCSE Chemistry Paper 2 2015
KCSE Chemistry Paper 2 2013
KCSE Chemistry Paper 2 2014
KCSE Chemistry Paper 2 2015
KCSE Chemistry Paper 2 2016
KCSE Chemistry Paper 2 2017
KCSE Chemistry Paper 3
KCSE Chemistry Paper 3 2012
KCSE Chemistry Paper 3 2016
KCSE Chemistry Paper 3 2017
KCSE Chemistry Paper 3 Past Papers
KCSE Chemistry Past Papers
KCSE Chemistry Past Papers and Answers
KCSE Chemistry Past Papers Pdf
KCSE Chemistry Practical
KCSE Chemistry Practical 2015
KCSE Chemistry Practical 2016
KCSE Chemistry Practical Past Papers
KCSE Chemistry Practicals
KCSE Chemistry Practicals KCSE Chemistry Paper 1
KCSE Chemistry Question and Answer
KCSE Chemistry Questions and Answers
KCSE Chemistry Questions and Answers Ap Chemistry
KCSE Chemistry Revision
KCSE Chemistry Revision Notes
KCSE Chemistry Revision Papers
KCSE Chemistry Revision Questions
KCSE Chemistry Revision Questions and Answers
KCSE Chemistry Syllabus
KCSE ChemistryNotes
KCSE ChemistryPaper 1
KCSE ChemistryPaper 2
KCSE ChemistryPaper 2 Pdf
KCSE ChemistrySyllabus
KCSE Business Paper 1 2016
KCSE Business Past Papers
KCSE Business Studies Past Papers
KCSE Essay Questions in Betrayal in the City
KCSE Essays
KCSE Exam Papers 2018
KCSE Exam Papers Answers
KCSE Form 1 Chemistry Revision
KCSE Form 2 Chemistry Revision
KCSE Form 3 Chemistry Revision
KCSE Form 4 Chemistry Revision
KCSE Form Four Chemistry Revision
KCSE Form One Chemistry Revision
KCSE Form Three Chemistry Revision
KCSE Form Two Chemistry Revision
KCSE KCSE Past Papers KNEC
KCSE Leakage
KCSE Leakage Chemistry
KCSE Made Familiar Chemistry
KCSE Made Familiar Chemistry Pdf
KCSE Marking Scheme 2016
KCSE Marking Schemes
KCSE Marking Schemes 2017
KCSE Marking Schemes Pdf
KCSE Mock Exams
KCSE Mock Papers 2015
KCSE Mock Papers 2017
KCSE Mock Papers 2018
KCSE Mock Papers Pdf
KCSE Mock Papers Pdf 2018
KCSE Mock Papers Pdf KCSE Past Papers
KCSE Mocks 2017
KCSE Mocks 2018
KCSE Notes
KCSE Online Notes
KCSE Online Past Papers
KCSE Online Registration
KCSE Papers 2015
KCSE Papers and Marking Schemes | Exams
KCSE Past Papers
KCSE Past Papers 2007
KCSE Past Papers 2009
KCSE Past Papers 2010
KCSE Past Papers 2011
KCSE Past Papers 2011 Pdf
KCSE Past Papers 2012
KCSE Past Papers 2013
KCSE Past Papers 2013knec
KCSE Past Papers 2014
KCSE Past Papers 2014 Pdf
KCSE Past Papers 2015
KCSE Past Papers 2015 Marking Schemes
KCSE Past Papers 2015 Pdf
KCSE Past Papers 2016
KCSE Past Papers 2016 Pdf
KCSE Past Papers 2017
KCSE Past Papers 2017 Pdf
KCSE Past Papers 2018
KCSE Past Papers Chemistry
KCSE Past Papers Chemistry and Answers
KCSE Past Papers Chemistry Pdf
KCSE Past Papers Chemistry With Answers
KCSE Past Papers Chemistryand Answers
KCSE Past Papers Business Studies and Answers
KCSE Past Papers KCSE and Answers
KCSE Past Papers KCSE and Answers Free Mocks Online
KCSE Past Papers Marking Scheme
KCSE Past Papers Pdf Download
KCSE Past Papers Pdf Download KCSE 2013
KCSE Past Papers With Answers
KCSE Past Papers Woodwork and Answers
KCSE Prediction 2017
KCSE Prediction 2018
KCSE Prediction 2018 Pdf
KCSE Prediction Papers 2018
KCSE Prediction Questions
KCSE Prediction Questions 2018
KCSE Prediction Questions and Answers
KCSE Questions
KCSE Questions and Answers
KCSE Questions and Answers.
KCSE Questions on Chemistry
KCSE Results, Online Registration, KCSE Result Slip.
KCSE Revision
KCSE Revision Notes
KCSE Revision Notes Chemistry
KCSE Revision Notes Pdf
KCSE Revision Papers
KCSE Revision Papers 2014
KCSE Revision Papers With Answers
KCSE Revision Question for Chemistry
KCSE Revision Questions
KCSE Revision Questions and Answers
KCSE Revision | Secondary School | Text Books | Text Book Centre
KCSE Syllabus Pdf
KCSE Trial 2017
KCSE Trial Exams 2017
Kenya Secondary School Chemistry Syllabus
Kenya Secondary School Chemistry Syllabus Pdf
Kenya Secondary School ChemistrySyllabus Pdf
Kenya Secondary School Syllabus Pdf
Kenya-kcse-christian Religious Education Syllabus
Kenyaplex KCSE Past Papers
Kenyaplex Past Papers for Secondary
KLB Chemistry Book 1 Download
KLB Chemistry Book 1 Notes
KLB Chemistry Book 1 Pdf
KLB Chemistry Book 2
KLB Chemistry Book 2 Notes
KLB Chemistry Book 2 Notes Pdf
KLB Chemistry Book 2 Pdf
KLB Chemistry Book 3 Notes
KLB Chemistry Book 3 Pdf
KLB Chemistry Book 3 Pdf Download
KLB Chemistry Book 4 Notes
KLB Chemistry Book 4 Pdf
KLB Chemistry Book 4 Pdf Download
KLB Chemistry Book 4 Topics
KLB Chemistry Book One
KLB Chemistry Form 1
KLB Chemistry Form 1 Notes
KLB Chemistry Form 1 Pdf
KLB Chemistry Form 2
KLB Chemistry Form 2 Book
KLB Chemistry Form 2 Notes
KLB Chemistry Form 2 Pdf
KLB Chemistry Form 2 Pdf Download
KLB Chemistry Form 2 Schemes of Work
KLB Chemistry Form 3
KLB Chemistry Form 3 Notes
KLB Chemistry Form 3 Notes Pdf
KLB Chemistry Form 3 Pdf
KLB Chemistry Form 3 Pdf Download
KLB Chemistry Form 4
KLB Chemistry Form 4 Notes
KLB Chemistry Form 4 Pdf
KLB Chemistry Form Four
KLB Chemistry Form Four Notes
KLB Chemistry Form One
KLB Chemistry Form One Notes
KLB Chemistry Form Three
KLB Chemistry Form Three Notes
KLB Chemistry Form Two
KLB Chemistry Form Two Notes
KLB Chemistry Notes
KLB Chemistry Notes Form 4
KLB Chemistry Pdf
KLB ChemistryNotes
KLB ChemistryNotes Form 4
KLB ChemistryPdf
KNEC Chemistry Syllabus
KNEC Examiners Portal KNEC Website
KNEC Ict Past Papers
KNEC Past Papers for Colleges
KNEC Past Papers Free Download
KNEC Past Papers Free Downloads
KNEC Past Papers Pdf
KNEC Portal Confirmation
KNEC Portal KCSE Results
KNEC Portal KNEC Past Papers for Colleges Kasneb Past Papers
KNEC Revision Papers
KNEC Technical Exams Past Papers
Kusoma Chemistry Notes
Kusoma Chemistry Notes Pdf
Kusoma Notes Chemistry
Kusoma.co.ke
Kusoma.com Past Papers
Learner Guide for Cambridge IGCSE Chemistry
Longhorn Chemistry Book 3 Pdf
Made Familiar Chemistry
Made Familiar Chemistry Pdf
Made Familiar Chemistry Questions
Maktaba Tetea Notes
Marking Scheme KCSE Chemistry Past Papers
Math Form2 Note
Mcqs About Gaseous Exchange
Middle School Chemistry Bowl Chemistry Questions
Mock Past Papers 2017
Mock Past Papers With Answers
Mokasa Mock 2017
More Than 1800 Chemistry Questions and Answers to Help You Study
Multiple Choice Questions on Chemistry
Necta Chemistry Past Papers
Necta Chemistry Practicals
Necta ChemistryPast Papers
Necta ChemistryPracticals
Necta Form Four Past Papers
Necta Past Papers Form 4
Necta Past Papers Form 4 2016
Necta Past Papers Form Six
Necta Past Papers Form Two
Necta Questions and Answers
Necta Review Questions
Notes Chemistry Form 1
Notes Chemistry Form 2
Notes Chemistry Form 3
Notes Chemistry Form 3 Notes Pdf
Notes Chemistry Form 3 Syllabus
Notes Chemistry Form 4 Syllabus
Notes on Chemistry Studies
Notes Za Chemistry 4m 2
Notes Za Chemistry Form One
Notes Za Chemistry Form Three
O Level Chemistry Practical Experiments
O Level Chemistry Questions and Answers Pdf
Orm Three Chemistry Notes
Page Navigation
Papacambridge Chemistry IGCSE
Papers KNEC KCSE Online Past
Papers KNEC KCSE Results Past Papers
Past KCSE Papers
Past Paper Questions by Topic Chemistry
Past Papers 2014
Past Papers in Kenya
Pdf Chemistry Form 3
Pdf Chemistry Notes
Pdf Chemistry Notes Form 1
Pdf Chemistry Notes Form 2
Pdf Chemistry Notes Form 3
Pdf Chemistry Notes Form 4
Pdf Chemistry Notes Form Four
Pdf Chemistry Notes Form One
Pdf Chemistry Notes Form Three
Pdf Chemistry Notes Form Two
Pdf Form 1 Chemistry Questions and Answers
Pdf Form 2 Chemistry Questions and Answers
Pdf Form 3 Chemistry Questions and Answers
Pdf Form 4 Chemistry Questions and Answers
Pdf Form Four Chemistry Questions and Answers
Pdf Form One Chemistry Questions and Answers
Pdf Form Three Chemistry Questions and Answers
Pdf Form Two Chemistry Questions and Answers
Pdf Free KCSE Past Papers and Marking Schemes
Pdf" Revision Questions Chemistry Form 1
Practical Chemistry Experiments Pdf
Practical Chemistry Question and Answer Pdf
Pre Mocks 2018
Preliminary Chemistry
Primary and Secondary Tillage Implements Ppt
Pte KNEC Past Papers
Questions and Answers Pdf Chemistry Form 1
Questions and Answers Pdf Chemistry Form 2
Questions and Answers Pdf Chemistry Form 3
Questions and Answers Pdf Chemistry Form 4
Questions and Answers Pdf Chemistry Form Four
Questions and Answers Pdf Chemistry Form One
Questions and Answers Pdf Chemistry Form Three
Questions and Answers Pdf Chemistry Form Two
Questions Based to Introduction to Chemistry
Questions on Gaseous Exchange in Humans
Questions on Introduction to Chemistry
Questions to Ask in Chemistry Class
Questions to Confuse Your Chemistry Teacher
Quizlet Chemistry Test
Quizlet Test Questions
Qustions in Chemistry and Answers
Revision
Revision Chemistry Notes and Questions?
Revision Quiz for Chemistry for Form Three
S.1 Chemistry Questions
S.2 Chemistry Questions
S.3 Chemistry Questions
S.4 Chemistry Questions
Sample Essays on Betrayal in the City
School Chemistry Notes
Secondary Chemistry Notes
Secondary Chemistry Notes Pdf
Secondary ChemistryNotes Pdf
Senior 1 Chemistry Notes
Senior 2 Chemistry Notes
Senior 3 Chemistry Notes
Senior 4 Chemistry Notes
Senior 5 Chemistry Notes
Senior 6 Chemistry Notes
Senior Five Chemistry Notes
Senior Four Chemistry Notes
Senior One Chemistry Notes
Senior Six Chemistry Notes
Senior Three Chemistry Notes
Senior Two Chemistry Notes
Simple Scientific Questions
Smart Questions to Ask a Chemistry Teacher
Snab Chemistry Revision Notes
Southwest Mock Paper 2 2016 Chemistry Only
Spm Chemistry Revision Notes
Spm Notes
Success Chemistry Spm Pdf
Success ChemistrySpm Pdf
Summary of Chemistry Form 3
Tahossa Past Papers
To Motivate a Form 4 KCSE Student
To Motivate a Form 4 Student
Topical Revision Material
Tricky Chemistry Questions and Answers
Tricky Chemistry Questions for Adults
Tricky Chemistry Questions With Answers
Tricky Chemistry Quiz Questions
Two Chemistry Revision Questions
University Chemistry Volume 3 Openstax
University Chemistry Volume 3 Pdf
University Chemistry Volume 4 Pdf
Ur Revision Guide IGCSE Chemistry
What Are the Types of Gametes
Working of Excretory System
Www.Chemistry Form One Notes.com
Www.Chemistry From One KLB.com
Www.form 1 Chemistry.com
Www.form 2 Chemistry.com
Www.form 3 Chemistry.com
Www.form 4 Chemistry.com
Www.form Four Chemistry.com
Www.form One Chemistry.com
Www.form Three Chemistry.com
Www.form Two Chemistry.com
Www.kusoma Notes
Www.kusoma Revision Materials
Www.kusoma.co.ke Chemistry Notes
Xtremepapers IGCSE Chemistry
Year 11 Chemistry
Z Notes Chemistry IGCSE
Znotes as Chemistry
Acids and Bases Chem Revision Questions
Chemistry Notes Combine Form 1, 2, 3 and 4
Chemistry Notes Form 3
A Level Chemistry Questions and Answers Pdf
All Chemistry Questions and Answers Pdf,ppt
Chemistry Exam Questions and Answers Pdf
Chemistry Multiple Choice Questions and Answers Pdf
Chemistry Questions and Answers for High Schools Pdf
Chemistry Notes Form 1-4 Pdf
Free High School Notes Kenya
Free Kcse Revision Notes
General Chemistry Test Questions and Answers Pdf
High School Chemistry Multiple Choice Questions and Answers Pdf
Kcse Chemistry Revision Notes Pdf
Kcse Revision Books Pdf
Kcse Revision Notes Pdf
Kenya Secondary School Notes Pdf
Notes of Form 123 and 4 All Subject
Chemistry Notes Form 1 Free Download
Advice to KCSE Candidates
Best Revision Books for KCSE
How to Pass an Exam Successfully
How to Pass KCSE 2018
How to Pass KCSE 2019
How to Pass KCSE Chemistry Paper
How to Pass KCSE Chemistry
How to Pass KCSE Chemistry
How to Pass KCSE 2019
K.c.s.e Chemistry Paper 1 2017
KCSE 2019 Prediction
KCSE Prediction 2019
KCSE Revision Tips
KCSE Chemistry Paper 1 2018
KCSE Chemistry Paper 2 2018
KCSE Chemistry Past Papers Pdf
KCSE Past Papers 2012
KCSE Past Papers 2017 Pdf
KCSE Past Papers 2018
KCSE Past Papers of Chemistry Pp2
KCSE Chemistry Past Papers Pdf
Chemistry KCSE Papers With Their Marking Schemes
Chemistry Paper 1 and Answers
KCSE 2017 Papers and Marking Scheme
KCSE 2019 Papers and Marking Scheme
KCSE Chemistry Paper 1 2018
KCSE Chemistry Paper 1 2019
KCSE Chemistry Paper 2 2017
KCSE Chemistry Paper 2 2018
KCSE Chemistry Paper 2 2019
KCSE Chemistry Past Papers and Answers
KCSE Marking Schemes Pdf
KCSE Past Papers 2015 Marking Schemes
KCSE Past Papers 2019 Marking Schemes
Chemistry KCSE Papers With Their Marking Schemes
Chemistry Paper 1 and Answers
KCSE 2017 Papers and Marking Scheme
KCSE 2019 Papers and Marking Scheme
KCSE Chemistry Paper 1 2018
KCSE Chemistry Paper 1 2019
KCSE Chemistry Paper 1 2019 Past Papers
KCSE Chemistry Paper 2 2017
KCSE Chemistry Paper 2 2018
KCSE Chemistry Paper 2 2019
KCSE Chemistry Paper 2 2019 Past Papers
KCSE Chemistry Paper 3 2019 Past Papers
KCSE Chemistry Past Papers and Answers
KCSE Marking Schemes Pdf
KCSE Past Papers 2015 Marking Schemes
KCSE Past Papers 2019 Marking Schemes
KCSE Past Papers Chemistry Paper 1 2019
KCSE Past Papers Chemistry Paper 2 2019
KCSE Past Papers Chemistry Paper 3 2019
Past Papers KCSE Chemistry Paper 1 2019
Past Papers KCSE Chemistry Paper 2 2019
Past Papers KCSE Chemistry Paper 3 2019
Chemistry Form 1 Questions and Answers
Chemistry Paper 1 2018
Chemistry Paper 2 2018
Chemistry Paper 1 2019
Chemistry Paper 2 2019
Chemistry Paper 1 Notes
Chemistry Paper 1 Questions and Answers Pdf
Chemistry Paper 2 Questions and Answers
Chemistry Questions and Answers Form 2
KCSE Chemistry Paper 1 2018
KCSE Chemistry Paper 2 2018
KCSE Chemistry Paper 1 2017
KCSE Chemistry Paper 2 2017
KCSE Chemistry Paper 1 2019
KCSE Chemistry Paper 2 2019
Most Tested KCSE Chemistry Questions
4m1 Notes Viusasa
4m2 Notes Viusasa
4m3 Notes Viusasa
4m4 Notes Viusasa
Download on Viusasa - Download Now for Free
Elimu - Viusasa
Elimu Library | Notes, Exams, Lesson Plans, Schemes
Elimu Online
High School Notes - Revision Materials for Kenyan Schools
Https //www.viusasa.com/elimu Kenya
Notes Viusasa Elimu Form 1
Notes Viusasa Elimu Form 2
Notes Viusasa Elimu Form 3
Notes Viusasa Elimu Form 4
Notes Viusasa Elimu Form Four
Notes Viusasa Elimu Form One
Notes Viusasa Elimu Form Three
Notes Viusasa Elimu Form Two
Viusasa
Viusasa Education
Viusasa Elimu
Viusasa Elimu Class 6
Viusasa Elimu Form 1
Viusasa Elimu Form 1 Notes
Viusasa Elimu Form 2
Viusasa Elimu Form 2 Notes
Viusasa Elimu Form 3
Viusasa Elimu Form 3 Notes
Viusasa Elimu Form 4
Viusasa Elimu Form 4 Notes
Viusasa Elimu Form Four
Viusasa Elimu Form Four Notes
Viusasa Elimu Form One
Viusasa Elimu Form One Notes
Viusasa Elimu Form Three
Viusasa Elimu Form Three Notes
Viusasa Elimu Form Two
Viusasa Elimu Form Two Notes
Viusasa High School Notes - Revision Materials for Kenyan Schools
Viusasa Notes
Chemistry Mock Papers
Chemistry Paper 1 2019
Chemistry Paper 1 Notes
Chemistry Paper 1 Questions and Answers
Chemistry Paper 1 Questions and Answers Pdf
Chemistry Paper 2 Form 3
Chemistry Paper 2 Notes
Chemistry Paper 2 Questions and Answers Pdf
Chemistry Past Papers
Chemistry Past Papers Pdf
Chemistry Questions and Answers Pdf
Chemistry Revision Questions and Answers Pdf
Chemistry Revision Questions Pdf
Common Exam Questions in Chemistry Paper 1
Common Exam Questions in Chemistry Paper 2
Common Test Questions in Chemistry Paper 1
Common Tested Questions in Chemistry Paper 1
Commonly Tested Questions in Chemistry Paper 1
K.c.s.e.Chemistry Questions and Answers
KCSE 2019 Chemistry Paper 1 Marking Scheme
KCSE 2019 Chemistry Paper 2
KCSE 2019 Chemistry Paper 2 Marking Scheme
KCSE 2020 Prediction Questions and Answers
KCSE Chemistry Paper 1 2019
KCSE Chemistry Paper 2 2016
KCSE Chemistry Paper 2 2017
KCSE Chemistry Paper 2 2018
KCSE Chemistry Paper 2 2019
KCSE Chemistry Questions and Answers
Most Tested Questions in Chemistry Paper 1
Most Tested Questions in Chemistry Paper 2
Mostly Tested Questions in Chemistry Paper 1
Mostly Tested Questions in Chemistry Paper 2
Chemistry Form 4
Chemistry Revision Questions Form 1
Combined Science Notes Form 1
Form 4 Notes
High Flyer Series Chemistry Form 1-4
Kcse Past Papers Chemistry With Answers
Chemistry Notes Download
Secondary Chemistry Notes Pdf
Water and Hydrogen Form 1 Notes
High Flyer Series KCSE Revision in Chemistry
High Flyer Series KCSE Revision Chemistry Form 1-4 Revised
A Level Chemistry Notes Uganda Pdf Download
Buddo Junior School Holiday Work
Chemistry Notes for a Level Pdf
Chemistry Notes Form 1
Chemistry Notes Form 3 the Mole
Chemistry Notes Form 4
Chemistry Notes O Level
Chemistry Notes O Level Uganda
Chemistry Notes O Level Uganda Pdf
Chemistry Notes Pdf
Download Chemistry Notes
Form 3 Chemistry Notes
Form 3 Chemistry Questions and Answers
Form Three Chemistry Syllabus Pdf
Gayaza High School Chemistry Notes
Gayaza High School Chemistry Notes Pdf
Gayaza High School Chemistry Past Papers
Gayaza High School Chemistry Past Papers Answers
Gayaza High School Chemistry Past Papers Questions and Answers
Gayaza High School E Learning
Gayaza High School Elearning Platform
Gayaza High School Examinations
Gayaza High School Holiday Work
Gayaza High School Notes
Gayaza High School Notes 2021
Gayaza High School Notes 2022
Gayaza High School Notes 2023
Gayaza High School Notes Pdf
Gayaza Junior School E Learning Platform
Gayaza Junior School Holiday Work
Gayaza O Level Chemistry Notes
Home › Gayaza High School | Elearning Platform
Kabojja Junior School Holiday Work Pdf
Klb Chemistry Book 3
Klb Chemistry Form 3 Teachers Guide
Klb Chemistry Notes
List of Ugandan E-learning Platforms for Students
Mirembe Junior School Holiday Work
Ntare School Past Papers
O Level Chemistry Notes Free Download
O Level Chemistry Notes in Uganda
O Level Chemistry Notes Pdf
O Level Chemistry Notes Uganda Pdf
O Level Chemistry Notes Uganda Pdf Download
O Level General - Home › Gayaza High School | Elearning
S.1 Chemistry Notes | Standard High School Zzana
S.3 Chemistry Notes
S.4 Chemistry 2 Notes | Standard High School Zzana
Seeta High School Elearning
Seeta High School Holiday Work
Seeta High School Notes
Seeta High School Notes Pdf
Seeta High School Past Papers
Seeta High School Past Papers Pdf
Senior 1 Chemistry Notes
Senior 1 Chemistry Notes in Uganda
Senior 1 Chemistry Notes Uganda
Senior 1 Chemistry Questions
Senior 1 Exams
Senior 1 Work
Senior 1 Work 2020
Senior 1 Work 2020 Uganda
Senior 2 Chemistry Notes
Senior 2 Chemistry Notes in Uganda
Senior 2 Chemistry Notes Uganda
Senior 2 Chemistry Questions
Senior 2 Exams
Senior 2 Work
Senior 2 Work 2020
Senior 2 Work 2020 Uganda
Senior 3 Chemistry Notes
Senior 3 Chemistry Notes in Uganda
Senior 3 Chemistry Notes Uganda
Senior 3 Chemistry Questions
Senior 3 Exams
Senior 3 Work
Senior 3 Work 2020
Senior 3 Work 2020 Uganda
Senior 4 Chemistry Notes
Senior 4 Chemistry Notes in Uganda
Senior 4 Chemistry Notes Uganda
Senior 4 Chemistry Questions
Senior 4 Exams
Senior 4 Work
Senior 4 Work 2020
Senior 4 Work 2020 Uganda
Senior Four Chemistry Notes
Senior Four Chemistry Notes in Uganda
Senior Four Chemistry Notes Uganda
Senior Four Chemistry Questions
Senior Four Exams
Senior Four Work
Senior Four Work 2020
Senior Four Work 2020 Uganda
Senior One Chemistry Notes
Senior One Chemistry Notes in Uganda
Senior One Chemistry Notes Uganda
Senior One Chemistry Questions
Senior One Exams
Senior One Work
Senior One Work 2020
Senior One Work 2020 Uganda
Senior Three Chemistry Notes
Senior Three Chemistry Notes in Uganda
Senior Three Chemistry Notes Uganda
Senior Three Chemistry Questions
Senior Three Exams
Senior Three Work
Senior Three Work 2020
Senior Three Work 2020 Uganda
Senior Two Chemistry Notes
Senior Two Chemistry Notes in Uganda
Senior Two Chemistry Notes Pdf
Senior Two Chemistry Notes Uganda
Senior Two Chemistry Questions
Senior Two Exams
Senior Two Work
Senior Two Work 2020
Senior Two Work 2020 Uganda
St Mary's Kitende E Learning
Standard High School Notes
Standard High School Zana a Level Notes
Standard High School Zana Com Notes
Standard High School Zana E Learning
Standard High School Zana E Learning Platform
Standard High School Zana E-learning Platform
Standard High School Zana Notes for Senior Two
Standard High School Zana Notes Pdf
Standard High School Zana Website
Standard High Zana Notes
Uace Chemistry Notes
Uace Chemistry Notes Pdf
Uce Chemistry Notes Pdf
Uce Chemistry Notes Pdf Download
Uce Past Papers
Uganda Secondary Schools E-learning Platform
Uneb Marking Guides Pdf
Uneb Past Papers and Answers Pdf
Air and Combustion Form 1 Notes
Chemistry Form 1 Notes Pdf Free Download
Chemistry Form One Questions and Answers
Chemistry Notes Form 1 Easy Elimu
Chemistry Notes Form 1-4 Pdf
Chemistry Notes Form 1-4 Pdf Download Free
High School Chemistry Notes Pdf
Klb Chemistry Form 1 Notes Pdf Download
Notes of Form One Chemistry Laboratory and Drawings
Chemistry Form 1 Questions and Answers Pdf Download
Chemistry Notes Form 1-4
Chemistry Form 1 Questions and Answers Pdf
Form 1 Chemistry Topical Questions
Form 1 Chemistry Exam Paper With Answer Pdf
Form 1 Exams 2020
Form 1 Chemistry Past Papers
Form 1 Chemistry Revision Papers With Answers
Sample Chemistry Test for Form One Exams
Ntare School Past Papers
Seeta High School Notes Pdf
Seeta High School Past Papers
Seeta High School Past Papers Pdf
St Mary's Kitende Past Papers
Uce Chemistry Notes Pdf
Uce Past Papers
Uneb Marking Guides Pdf
Uneb Past Papers and Answers Pdf
Chemistry Book 3 Download
Chemistry Form 3 Questions and Answers
Chemistry Form 3 Syllabus
Chemistry Form 3 Topics
Chemistry Notes Form 3
Chemistry Notes Form 3
Chemistry Notes Form 3
Chemistry Notes Form Three
Chemistry Notes Pdf Download
Form 3 Chemistry Exam
Form 3 Chemistry Notes on Pdf
Revision Chemistry Revision a Level Chemistry
Revision Notes - Chemistry
S.1-Chemistry-notes
S.1-Chemistry-notes Uganda
S.2-Chemistry-notes
S.2-Chemistry-notes Uganda
S.3-Chemistry-notes
S.3-Chemistry-notes Uganda
S.4-Chemistry-notes
S.4-Chemistry-notes Uganda
S.5-Chemistry-notes
S.5-Chemistry-notes Uganda
S.6-Chemistry-notes
S.6-Chemistry-notes Uganda
S1 Chemistry Notes Term 1
S1 Chemistry Notes Term 2
S1 Chemistry Notes Term 3
S2 Chemistry Notes Term 1
S2 Chemistry Notes Term 2
S2 Chemistry Notes Term 3
S3 Chemistry Notes Term 1
S3 Chemistry Notes Term 2
S3 Chemistry Notes Term 3
S4 Chemistry Notes Term 1
S4 Chemistry Notes Term 2
S4 Chemistry Notes Term 3
Senior 3 Chemistry Notes
Senior 3 Chemistry Notes Uganda
Senior Three Chemistry Notes
Senior Three Chemistry Notes Uganda
Chemistry Form 1 the Cell
Chemistry Form One Notes Free – Education News
Chemistry Notes Form 1-4
Chemistry Questions and Answers Form 1 - Chemistry Form One
Chemistry Questions and Answers Form 2 - Chemistry Form Two
Chemistry Questions and Answers Form 3 - Chemistry Form Three
Chemistry Questions and Answers Form 4 - Chemistry Form Four
Chemistry Questions and Answers Senior 1 - Chemistry Senior One
Chemistry Questions and Answers Senior 2 - Chemistry Senior Two
Chemistry Questions and Answers Senior 3 - Chemistry Senior Three
Chemistry Questions and Answers Senior 4 - Chemistry Senior Four
Chemistry Topic by Topic Questions and Answers
Combined Science Notes Form 1
Form 1 Chemistry Revision Questions and Answers
Form 1 Chemistry Topical Questions
Form 1 Revision Papers With Answers
Form 2 Chemistry Revision Questions and Answers
Form 3 Chemistry Revision Questions and Answers
Form Four Chemistry Revision Questions and Answers
Form One Chemistry Revision Questions and Answers
Form Three Chemistry Revision Questions and Answers
Form Two Chemistry Revision Questions and Answers
Free Chemistry Form 1 Notes
Free Chemistry Notes
Introduction to Chemistry Form One
Kcse Past Papers: Chemistry Form 1
Kcse Past Papers: Chemistry Form 1 Topical Questions
Kcse Past Papers: Chemistry Form 1 Topical Questions and Answers
Most Tested Areas in Chemistry Kcse
Most Tested Areas in Chemistry Kcse Exams
Most Tested Areas in Chemistry Uneb
Most Tested Areas in Form 1 in Chemistry
Most Tested Areas in Form 2 in Chemistry
Most Tested Areas in Form 3 in Chemistry
Most Tested Areas in Form 4 in Chemistry
Most Tested Areas in Form Four in Chemistry
Most Tested Areas in Form One in Chemistry
Most Tested Areas in Form Three in Chemistry
Most Tested Areas in Form Two in Chemistry
Most Tested Areas in Kcse Chemistry
Most Tested Areas in Kcse Chemistry Exams
Most Tested Areas in Senior 1 in Chemistry
Most Tested Areas in Senior 2 in Chemistry
Most Tested Areas in Senior 3 in Chemistry
Most Tested Areas in Senior 4 in Chemistry
Most Tested Areas in Senior Four in Chemistry
Most Tested Areas in Senior One in Chemistry
Most Tested Areas in Senior Three in Chemistry
Most Tested Areas in Senior Two in Chemistry
Most Tested Areas in Uneb Chemistry
Revision Notes Chemistry Form 1 - Free Kcse Past Papers
Who Was Chemistry Champion KCSE 2019
Top 100 Students in Chemistry KCSE 2019
Best 100 Students in Chemistry KCSE 2019
Top Student in Chemistry KCSE
Best Student in Chemistry KCSE
Who Was Chemistry Champion KCSE 2020
Top 100 Students in Chemistry KCSE 2020
Best 100 Students in Chemistry KCSE 2020
Names of Best Chemistry Students KCSE
Names of Top Chemistry Students KCSE
High School Chemistry Notes Pdf
Best High School Chemistry Notes Pdf
Comprehensive High School Chemistry Notes Pdf
Klb Chemistry Form 1 Book Pdf
Chemistry Form 1 Best Notes
Chemistry Form 1 Notes Pdf
Chemistry Form 1 Pressure
Chemistry Form 1 Questions and Answers
Chemistry Form 1 Questions and Answers Pdf Download
Chemistry Form 2 Best Notes
Chemistry Form 3 Best Notes
Chemistry Form 4 Best Notes
Chemistry Form Four Best Notes
Chemistry Form One Best Notes
Chemistry Form Three Best Notes
Chemistry Form Two Best Notes
Chemistry Notes Form 1 to 4
Chemistry Notes Pdf
Scholarship 2026/27
Current Scholarships 2026/2027 - Fully Funded
Full Undergraduate Scholarships 2026 - 2027
Fully Funded Masters Scholarships 2026 - 27
PhD Scholarships for International Students - Fully Funded!
Funding Opportunities for Journalists 2026/2027
Funding for Entrepreneurs 2026/2027
***
