KCSE Past Papers 2021 Chemistry paper 2(233/2)
Chemistry - Paper 2 2021 - 2 Hours
2021 Chemistry paper 2
1. Table 1 gives the properties of two compounds, A and B.
a. Table 1
| A |
B |
|---|---|
| white, crystalline, efflorescent |
white, crystalline, deliquescent |
State and explain the observation made when each of the compounds is left exposed in air: i. Compound A (2 marks)
ii. Compound B (2 marks)
b. In an experiment to determine the formula of hydrated magnesium sulphate, a sample was heated in a crucible until a constant mass was obtained. The results are shown in Table 2.
| Mass of crucible |
25.62 g |
|---|---|
| Mass of crucible + solid before heating |
28.08 g |
| Mass of crucible + solid after heating |
26.82 g |
Using the information in Table 2, determine the formula of the hydrated salt (Mg - 24.0; S = 32.0; O=16.0; H = 1.0). (3 marks)
c. Figure 1 shows analysis of an alloy containing two metals.
| Time (se conds) |
0 |
30 |
60 |
90 |
120 |
150 |
180 |
210 |
240 |
|---|---|---|---|---|---|---|---|---|---|
| Volume Of CO2 (cm3) |
O |
62 |
92 |
113 |
124 |
130 |
132 |
133 |
133 |
(Mg - 24.0; S = 32.0; O=16.0; H = 1.0). (3 marks)
c. Figure 1 shows analysis of an alloy containing two metals.
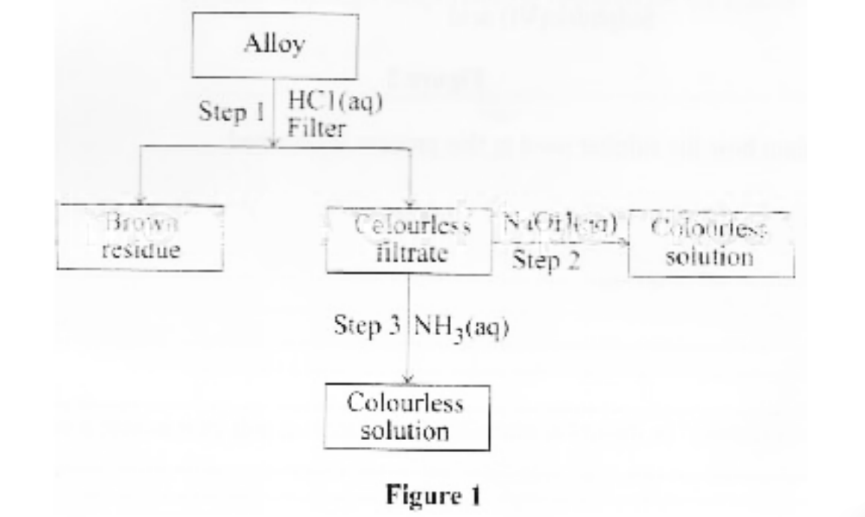
ii. Write the formula of the complex ion present in the colourless solution obtained in step 2 (1 mark)
iii. Identify the metals in the alloy, (2 marks)
2. The flow chart in Figure 2 shows the processes involved in the manufacture of sulphuric(VI) acid.
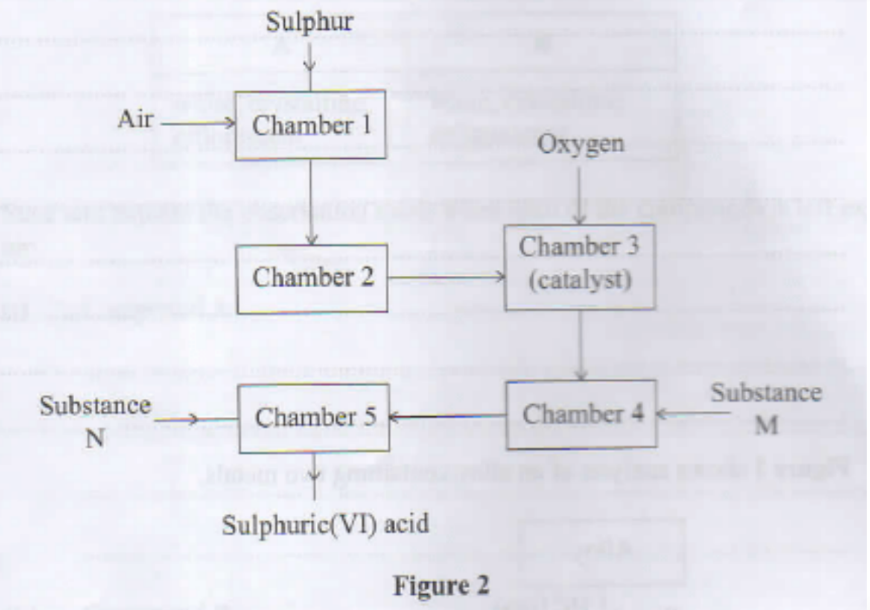
b. Give one advantage of using air in chamber 1 instead of using oxygen gas. (1 mark)
c. Identify substances: (1)
i. M (1 mark)
ii. N (1 mark)
d.i. In chamber 2, drying and purification take place. Give a reason why this is necessary (1 mark)
ii. The reaction in chamber 3 is highly exothermic.
I. Explain why high temperature is required for the reaction in chamber 3. (1 mark)
II. State how heat produced in chamber 3 can be utilised in this process e. Give a reason why this method of manufacture is known as 'contact process". (1 mark)
f. Emission of gases in the sulphuric(VI) acid plant may lead to environmental pollution.
i. State the evidence that could be used to show that the sulphuric(VI) acid plant causes pollution (1 mark)
ii. Explain how the pollution identified in 2(1)(i) can be controlled. (1 mark)
3.a. Chemical reactions occur as a result of collisions of particles. Give a reason why not all collisions are effective. (1 mark)
b. State and explain how the following factors affect the rate of reaction: i. Surface area of reactants. (1 mark)
ii. Pressure. (1 mark)
c. In an experiment to determine the rate of a reaction, marble chips were added to excess 2M hydrochloric acid. The equation for the reaction is:
CaCO3(s) + 2HCl(aq) → CaCI2(aq) + CO2(g) + H2O(l)
The volume of carbon(IV) oxide produced was measured at 25°C and recorded after every 30 seconds.
Table 3 shows the results obtained.
| Time (se conds) |
0 |
30 |
60 |
90 |
120 |
150 |
180 |
210 |
240 |
|---|---|---|---|---|---|---|---|---|---|
| Volume Of CO2 (cm3) |
O |
62 |
92 |
113 |
124 |
130 |
132 |
133 |
133 |
I. 45th second. (1 mark)
II. 105th second. (1 mark)
iii. Give a reason for the differences in the two rates. (1 mark)
iv. Using the graph, determine the mass of marble chips that reacted (2 marks)
(Ca=40.0; C = 12.0; 0 - 16.0;)
Molar gas volume at room temperature and pressure = 24000 cm").
4.a. Sea water contains approximately 3% sodium chloride. Describe how sodium chloride is obtained from sea water. (3 marks)
b. The solubility of sodium chloride is 36.2 g in 100 g of water at room temperature.
Determine the concentration in moles per litre of a saturated aqueous sodium chloride at room temperature (Na= 23.0; Cl= 35.5; density of water = 1.0 gem"). (2 marks)
c. Ammonia is highly soluble in water.
i. Explain how aqueous ammonia is prepared starting with ammonia gas. (2 marks)
ii. On the axes provided, sketch a curve showing how solubility of ammonia gas varies with temperature. (1 mark)

d. Water hardness is due to the presence of magnesium and calcium ions. Explain how these ions get into sources of water. (2 marks)
5.a. Figure 3 shows part of a Periodic Table.
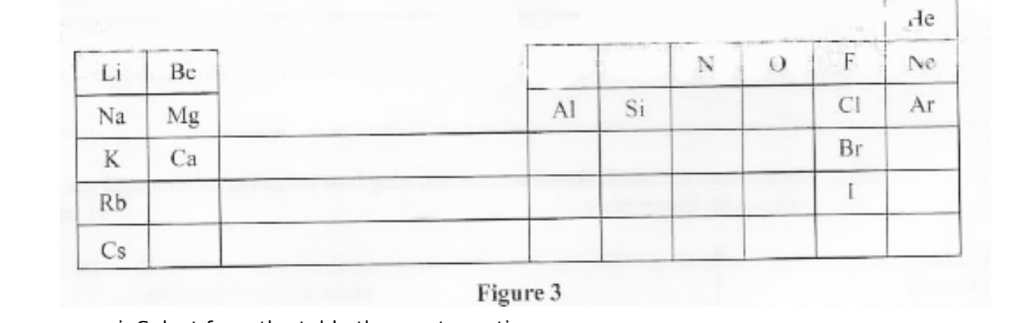
II. non-metal. (1⁄2 mark)
ii. Select an element with the highest first ionisation energy. (1 mark)
iii.I. Name the method used to obtain argon from its source. (1 mark)
II. Give one industrial use of argon. (1 mark)
iv. Explain each of the following observations:
I. The melting point of lithium is higher than that of potassium. (1 mark)
II. The melting point of chlorine is lower than that of iodine. (1 mark)
v. The following ions have the same number of electrons: N2-, Mg2+, 02, Na+ Arrange them in order of increasing ionic size. Give a reason for the order. (2 marks)
b. Use Table 4 to answer the questions that follow.
| Properly | Substance | |||
|---|---|---|---|---|
| H | I | J | K | |
| Melting point (°C) |
993 |
113 |
-38.9 |
-85 |
| Boiling point (°C) |
1695 |
183 |
357 |
-60 |
| Electrical conductivity at room temperature |
Does not conduct |
Does not conduct |
Conducts |
Does not conduct |
| Electrical conductivity in molten state |
Conducts |
Does not conduct |
Conducts |
Does not conduct |
ii. Name the particles responsible for electrical conductivity in substance: IH (1 mark)
I. H II. J (1 mark)
iii. Identify the type of forces that hold the particles together in: I. H (1 mark)
II. Κ (1 mark)
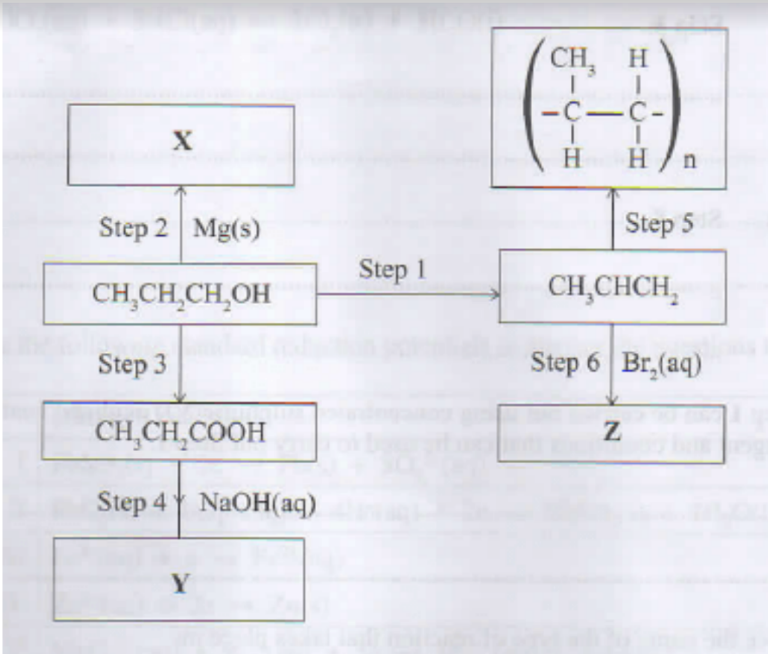
6. Figure 4 shows a flow chart involving reactions of some organic compounds.
Name:
Formula:
ii. Y
Name:
Formula:
b. Give the reagents and conditions necessary for carrying out:
i. Step 3. (1 mark)
ii. Step 5. (1 mark)
i. Write an equation for the reaction in step 6. (1 mark)
ii. State the observations made in step 6. (1 mark)
7.a. Using the oxidation numbers of chlorine, explain why the following is a redox reaction. HCIO3(aq) + 5HCl(aq) → 3CI2(g) + 3H2O(I) (2 marks)
b. Use the following standard reduction potentials to answer the questions that follow:
| I |
Half cell reactions |
Eo/V |
|---|---|---|
| II |
PSO4 (s) + 2e → Pb(s) + SO4'-(aq) |
-0.36 |
| III |
PBO2(s) + S042- (aq) + 4H+(aq) + 2e → PbSO4 (s) + 2HyO(1) |
+1.69 |
| IV |
Fe3+(aq) + e → Fe2+(aq) |
+0.77 |
| V |
Zn2+(aq) + 2e → Zn(s) |
-0.76 |
| V |
MnO4-' (aq) + 8H+(aq) + 5e -+ Mn2 (aq) + 4HtO(I) |
+1.51 |
| Vl |
O2g+ 2 H -I- (aq) -- 2e —+ H2O2(aq) |
+0.68 |
| VII |
Fe2+(aq) -i- 2e → Fe(s) |
-0.44 |
| VIII |
Ca2+(aq) → 2e Cu(s) |
+0.34 |
i. The half cells I and II are combined to form an electrochemical cell.
I. Write an equation for the cell reaction. (1 mark)
II. Calculate the e.m.f of the cell. (1 mark)
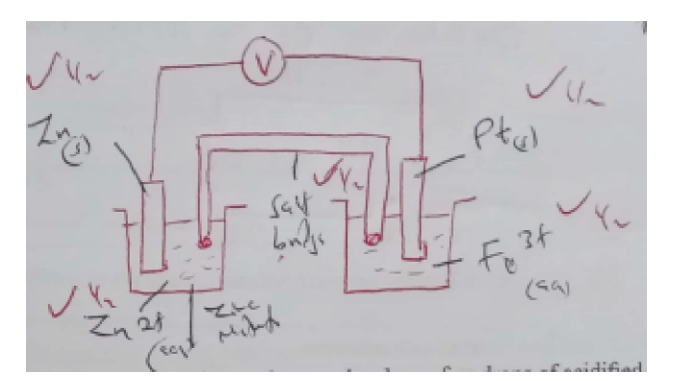
ii. Draw a labelled diagram for the electrochemical cell formed using half cells III and IV. (3 marks)
iii. State and explain the observations made when a few drops of acidified potassium manganate(VII) are added to hydrogen peroxide. (3 marks)
iv. Coating iron with zinc is a more effective way of corrosion prevention than coating it with copper. Explain. (2 marks)
Questions and Answers
Kenya certificate of Secondary Education
2021 Chemistry paper 2
1. Table 1 gives the properties of two compounds, A and B.
a. Table 1
1.
| A |
B |
|---|---|
| white, crystalline, efflorescent |
white, crystalline, deliquescent |
State and explain the observation made when each of the compounds is left exposed in air:
i. Compound A (2 marks)
ii. Compound B (2 marks)
b. In an experiment to determine the formula of hydrated magnesium sulphate, a sample was heated in a crucible until a constant mass was obtained. The results are shown in Table 2. Table 2
| Mass of crucible |
25.62 g |
|---|---|
| Mass of crucible + solid before heating |
28.08 g |
| Mass of crucible + solid after heating |
26.82 g |
Using the information in Table 2, determine the formula of the hydrated salt (Mg - 24.0; S = 32.0; O=16.0; H = 1.0). (3 marks)
MgSO4 H2O
Mass(g) 1.20 1.26
Moles 1.20 1.26
120 18 0.07/0.01
0.01/0.01
1 7
MgSO4.7H2O
c. Figure 1 shows analysis of an alloy containing two metals.
| Time (se conds) |
0 |
30 |
60 |
90 |
120 |
150 |
180 |
210 |
240 |
|---|---|---|---|---|---|---|---|---|---|
| Volume Of CO2 (cm3) |
O |
62 |
92 |
113 |
124 |
130 |
132 |
133 |
133 |

ii. Write the formula of the complex ion present in the colourless solution obtained in step 2 (1 mark)
iii. Identify the metals in the alloy, (2 marks)
2. The flow chart in Figure 2 shows the processes involved in the manufacture of sulphuric(VI) acid.

b. Give one advantage of using air in chamber 1 instead of using oxygen gas. (1 mark)
c. Identify substances: (1)
i. M (1 mark)
ii. N (1 mark)
d.i. In chamber 2, drying and purification take place. Give a reason why this is necessary (1 mark)
ii. The reaction in chamber 3 is highly exothermic.
I. Explain why high temperature is required for the reaction in chamber 3. (1 mark)
II. State how heat produced in chamber 3 can be utilised in this process
e. Give a reason why this method of manufacture is known as 'contact process". (1 mark)
Reactants come in contact with catalyst
f. Emission of gases in the sulphuric(VI) acid plant may lead to environmental pollution.
i. State the evidence that could be used to show that the sulphuric(VI) acid plant causes pollution (1 mark)
ii. Explain how the pollution identified in 2(1)(i) can be controlled. (1 mark)
Passing through Ca(OH)2/CaO
Scrubbing
3.a. Chemical reactions occur as a result of collisions of particles. Give a reason why not all collisions are effective. (1 mark)
b. State and explain how the following factors affect the rate of reaction:
i. Surface area of reactants. (1 mark)
ii. Pressure. (1 mark)
c. In an experiment to determine the rate of a reaction, marble chips were added to excess 2M hydrochloric acid. The equation for the reaction is:
CaCO3(s) + 2HCl(aq) → CaCI2(aq) + CO2(g) + H2O(l)
The volume of carbon(IV) oxide produced was measured at 25°C and recorded after every 30 seconds.
Table 3 shows the results obtained.
| Time (se conds) |
0 |
30 |
60 |
90 |
120 |
150 |
180 |
210 |
240 |
|---|---|---|---|---|---|---|---|---|---|
| Volume Of CO2 (cm3) |
O |
62 |
92 |
113 |
124 |
130 |
132 |
133 |
133 |
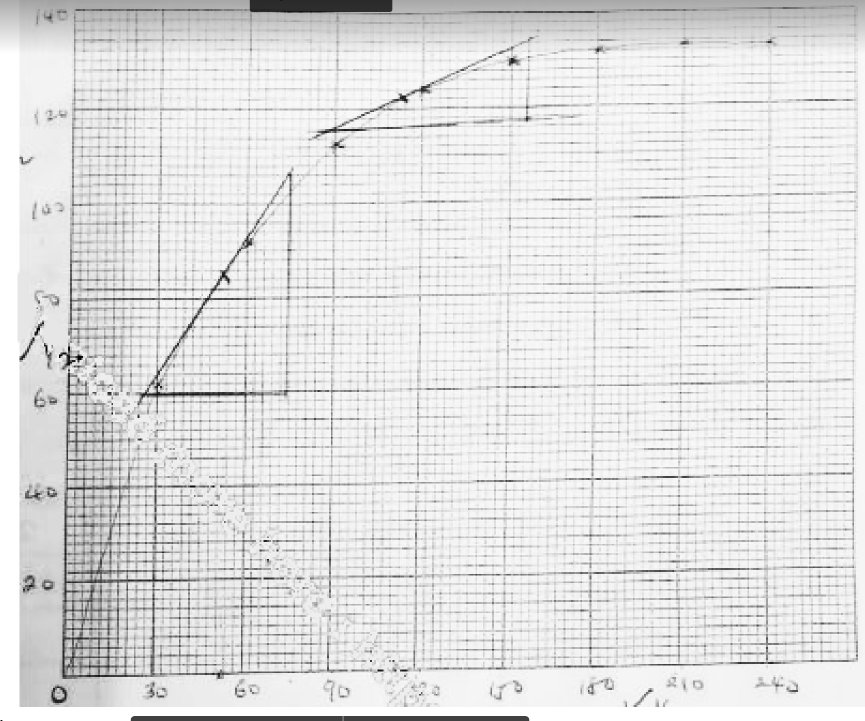
Tangent 45
Calculations from graph
dy
Ans = cm3/sec
II. 105th second. (1 mark)
Tangent 45
Calculations from graph
dy2/dx2 - dy1/dx1
Ans = cm3/sec
iii. Give a reason for the differences in the two rates. (1 mark)
iv. Using the graph, determine the mass of marble chips that reacted (2 marks) (Ca=40.0; C = 12.0; 0 - 16.0;) Molar gas volume at room temperature and pressure = 24000 cm").
Moles = 133/24000
Moles ratio 1:1
CaCO3
5.54 x 10-3 x 100 RFM
100 x 133 (over) 24000
Ans = 0.554g
4.a. Sea water contains approximately 3% sodium chloride. Describe how sodium chloride is obtained from sea water. (3 marks)
b. The solubility of sodium chloride is 36.2 g in 100 g of water at room temperature.
Determine the concentration in moles per litre of a saturated aqueous sodium chloride at room temperature (Na= 23.0; Cl= 35.5; density of water = 1.0 gem"). (2 marks)
RFM NaCl = 58.5
36.2 x 1000 (over) 100 = 362
362/58.5
6.188M or 6.19M
c. Ammonia is highly soluble in water.
i. Explain how aqueous ammonia is prepared starting with ammonia gas. (2 marks)
ii. On the axes provided, sketch a curve showing how solubility of ammonia gas varies with temperature. (1 mark)
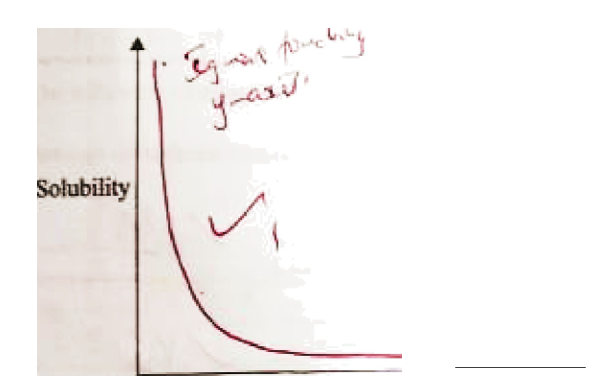
d. Water hardness is due to the presence of magnesium and calcium ions. Explain how these ions get into sources of water. (2 marks)
ions
5.a. Figure 3 shows part of a Periodic Table.

Cs
II. non-metal. (1⁄2 mark)
F
ii. Select an element with the highest first ionisation energy. (1 mark)
HI
I. Name the method used to obtain argon from its source. (1 mark)
Fractional distillation
II. Give one industrial use of argon. (1 mark)
Used in flourescent bulbs/lamps
iv. Explain each of the following observations:
I. The melting point of lithium is higher than that of potassium. (1 mark)
II. The melting point of chlorine is lower than that of iodine. (1 mark)
v. The following ions have the same number of electrons: N2-, Mg2+, 02-, Na+ Arrange them in order of increasing ionic size. Give a reason for the order. (2 marks)
Mg2+; Na+; O2-; N3-;
Protons decreases from Mg to nitrogen hence nuclear attraction decreases from Mg to N
b. Use Table 4 to answer the questions that follow.
| Properly | Substance | |||
|---|---|---|---|---|
| H | I | J | K | |
| Melting point (°C) |
993 |
113 |
-38.9 |
-85 |
| Boiling point (°C) |
1695 |
183 |
357 |
-60 |
| Electrical conductivity at room temperature |
Does not conduct |
Does not conduct |
Conducts |
Does not conduct |
| Electrical conductivity in molten state |
Conducts |
Does not conduct |
Conducts |
Does not conduct |
i. Identify the substance which is a gas at room temperature.
Give a reason. (1 mark)
K; boiling point below room temperature
ii. Name the particles responsible for electrical conductivity in substance: IH (1 mark)
I. H ions/ mobile ions
II. J (1 mark)
electrons/ delocalized electrons
iii. Identify the type of forces that hold the particles together in:
I. H (1 mark)
electrostatic forces/ ionic bonds
II. Κ (1 mark)
weak van der waals forces/ intermolecular
6. Figure 4 shows a flow chart involving reactions of some organic compounds.

Name:
i. Magnesium propoxide
Formula:
(CH3CH2CH2O)2Mg
ii. Y
Name:
Sodium propanoate
Formula:
CH3CH2COONa
b. Give the reagents and conditions necessary for carrying out:
i. Step 3. (1 mark)
H+/ KMnO4/H+K2Cr2O7
Warm/ Heat/ High temperature
ii. Step 5. (1 mark)
i. Write an equation for the reaction in step 6. (1 mark)
CH3CHCH2 + Br2 → CH3CHBr2
ii. State the observations made in step 6. (1 mark)
7.a. Using the oxidation numbers of chlorine, explain why the following is a redox reaction.
HCIO3(aq) + 5HCl(aq) → 3CI2(g) + 3H2O(I) (2 marks)
Oxidatopn of Cl in HClO from +5 to 0 Reduction -1 to 0 - Oxidation
b. Use the following standard reduction potentials to answer the questions that follow:
| I |
Half cell reactions |
Eo/V |
|---|---|---|
| II |
PSO4 (s) + 2e → Pb(s) + SO4'-(aq) |
-0.36 |
| III |
PBO2(s) + S042- (aq) + 4H+(aq) + 2e → PbSO4 (s) + 2HyO(1) |
+1.69 |
| IV |
Fe3+(aq) + e → Fe2+(aq) |
+0.77 |
| V |
Zn2+(aq) + 2e → Zn(s) |
-0.76 |
| V |
MnO4-' (aq) + 8H+(aq) + 5e -+ Mn2 (aq) + 4HtO(I) |
+1.51 |
| Vl |
O2g+ 2 H -I- (aq) -- 2e —+ H2O2(aq) |
+0.68 |
| VII |
Fe2+(aq) -i- 2e → Fe(s) |
-0.44 |
| VIII |
Ca2+(aq) → 2e Cu(s) |
+0.34 |
i. The half cells I and II are combined to form an electrochemical cell.
I. Write an equation for the cell reaction. (1 mark)
PbO2(s) + 2SO22-(aq) + 4H+ + Pb → 2PbSO4(s) +2H2O(I)
II. Calculate the e.m.f of the cell. (1 mark)
+1.69 = 0.36
+ 2.05V
ii. Draw a labelled diagram for the electrochemical cell formed using half cells III and IV. (3 marks)
H+/KMnO4 Decolorized/ Purple to colourless Effervescence/ Bubbles of colourless gas H2O2 oxidised to O2 gas/ Production of O2S4 MnO4-
Mn2+
iv. Coating iron with zinc is a more effective way of corrosion prevention than coating it with copper. Explain. (2 marks)
Secondary School Scholarships in Kenya » Kenya Postgraduate Scholarships » Undergraduate Scholarships for Kenyan Students » Kenya Scholarships for Kenyan Students Studying in Kenya » Kenya Undergraduate Scholarships » The Kenya Youth Education Scholarship Fund - Scholarships Kenya - Scholarships KCSE Results » KCSE Results Top 100 Schools - Kenya Certificate of Secondary Education – KCSE » KCSE Top 100 Candidates » Kenya Certificate of Secondary Education – KCSE » KNEC - Kenya National Examinations Council » Secondary Schools in Kenya » KNEC - Kenya National Examinations Council » Free KNEC KCSE Past Papers
Kenya Scholarships for Undergraduate Students » Kenya Scholarships for Postgraduate Students » Undergraduate Scholarships for Kenyan Students » Kenya Undergraduate Scholarships » Full Undergraduate Scholarships for Kenyans » Kenya Postgraduate Scholarships » Scholarships & Grants » Undergraduate Scholarships » Universities in Kenya » Kenya Universities and Colleges Central Placement Service (KUCCPS) » Colleges in Kenya » KASNEB Registration & Results » Secondary Schools Scholarships in Kenya » Undergraduate & Graduate Scholarships for Kenyans
Powerful Motivational Quotes for Students » Success Quotes for Students » KCSE Motivational Quotes for KCSE Candidates » KCSE Success Quotes for KCSE Candidates
1 a a kcse past papers 2014 kcse marking schemes 2016 kcse papers 2016 kcse prediction questions 2018 kcse exam 2018 kcse questions a a kcse past papers advance-africa.com kcse rev quiz agriculture mock papers agriculture paper 2 questions and answers pdf alliance mocks 2017 ap biology essay questions and answers arabic exam 2016 arabic oral exam questions betrayal in the city essay questions and answers pdf betrayal in the city essay questions with answers betrayal in the city, ,,revision questions biology book 3 klb biology essay questions and answers form 4 biology essay questions and answers form 4 pdf biology essays pdf biology exam questions and answers pdf biology form 2 questions and answers pdf biology form 3 notes pdf biology form 3 questions and answers pdf biology form 3 syllabus biology form three reproduction biology form three-questions and answers biology kcse - kcse biology questions and answers - kcse biology essay questions and answers - kcse biology paper 1 2015 - kcse biology notes - kcse 2015 biology paper 2 - kcse biology practical 2015 - kcse biology practicals - kcse biology 2011
biology kcse 2017 biology kcse questions biology paper 1 questions and answers biology paper 2 questions and answers biology paper 3 questions and answers biology questions and answers for high schools biology questions and answers for high schools pdf biology questions and answers form 2 biology questions and answers multiple choice biology questions and answers on cells biology questions and answers online biology questions and answers pdf biology revision notes form 3 business past kcse past papers c.r.e form one notes pdf cambridge igcse computer science cambridge igcse computer science answers cambridge igcse computer science coursebook pdf download cambridge igcse computer science revision guide pdf cambridge igcse computer science study and revision guide pdf cambridge igcse computer science workbook - free download cambridge igcse computer science workbook pdf caucasian chalk circle essay questions chemistry paper 1 questions and answers chemistry paper 2 questions and answers chemistry paper 3 question and answer chemistry past papers form 1 chemistry past papers form 2 cie past papers computer science 0478 computer science igcse past papers xtremepapers computer science paper 2 2017 computer science past papers a level computer science past papers o level computer studies form 1 questions computer studies form 3 past papers computer studies past papers computer studies questions and answers pdf county mocks 2017 cre form 2 notes pdf cre form 3 notes cre form 3 notes pdf cre form 4 notes cre form 4 notes pdf cre form one notes cre kcse 2016 cre notes cre notes form 2 cre notes pdf cre paper 1 with answers cre paper 2 cre paper 2 topics cre preparation notes cre questions form one cre revision notes cre revision questions and answers download kcse past papers with answers dvance kcse past papers edexcel igcse computer science past papers english paper 3 question paper - 2014 kcse english paper 3 question paper - 2015 kcse english paper 3 question paper - 2016 kcse english paper 3 question paper - 2017 kcse english paper 3 question paper - 2018 kcse essay questions and answers on betrayal in the city essay questions based on betrayal in the city find download kcse past papers with answers - kcse past papers pdf download - kcse 2013 marking scheme - kcse mathematics past papers pdf - free kcse past papers and marking schemes - kcse mock papers pdf - kcse past papers 2014 pdf - kcse past papers 2015 - kcse past papers 2010 find kcse biology essay questions and answers - kcse biology practicals - kcse biology paper 1 2015 - biology essay questions and answers form 4 - kcse biology questions and answers - ap biology essay questions and answers - kcse biology notes - kcse biology paper 2 2012 - kcse biology paper 2 2015
form 2 biology questions and answers free kcse mocks 2015 free kcse past papers - kcse past papers - knec kcse online past papers - knec kcse results past papers free kcse past papers 2014 free kcse past papers kenya, free marking schemes, download ... free kcse past papers with answers free kcse questions and answers on chemistry free revision papers general biology test questions and answers general science questions and answers pdf history and government paper one topics history form one questions and answers pdf history paper 1 questions and answers history paper 2 questions and answers home science past papers igcse computer science book igcse computer science book pdf download igcse computer science notes igcse computer science paper 2 notes igcse computer science past papers igcse computer science past papers 2014 igcse computer science past papers 2017 igcse computer science pdf igcse computer science pre release material 2018 igcse computer science resources igcse computer science revision notes pdf igcse computer science workbook pdf igcse computer studies past papers interesting biology questions ire kcse past papers k.c.s.e cre paper 1 2017 k.c.s.e geography 2017 k.c.s.e mathematics paper 1 2017 k.c.s.e mocks 2018 k.c.s.e past papers 2014 kcpe 2018 predictions kcpe prediction questions kcse 2010 marking scheme kcse 2010 past papers kcse 2011 cre paper 1 kcse 2011 marking scheme kcse 2012 history paper 2 marking scheme kcse 2012 marking schemes kcse 2013 cre paper 1 kcse 2013 marking scheme kcse 2013 marking scheme pdf kcse 2014 kcse 2015 biology paper 2 kcse 2015 biology paper 3 kcse 2015 marking scheme kcse 2015 past papers kcse 2016 agriculture paper 2 kcse 2016 biology paper 1 kcse 2016 biology paper 2 kcse 2016 computer paper 1 kcse 2017 marking scheme kcse 2017 maths paper 1 kcse 2017 papers kcse 2017 papers and marking scheme kcse 2017 past papers kcse 2017 prediction pdf kcse 2018 cre prediction kcse 2018 leakage kcse 2018 marking scheme kcse 2018 papers kcse 2018 predictions kcse 2019 marking scheme kcse agriculture past papers kcse answers kcse arabic paper 1 kcse arabic paper 2 kcse arabic paper 3 kcse arabic paper 3 2016 kcse arabic past papers kcse biology 2011 kcse biology essay questions and answers kcse biology essay questions and answers - kcse revision questions and answers - kcse chemistry questions and answers - kcse revision papers with answers - kcse past papers with answers - download kcse past papers with answers - kcse questions on the river and the source - kcse revision notes
kcse biology essay questions and answers - kcse revision questions and answers - kcse chemistry questions and answers - kcse revision papers with answers - kcse past papers with answers - download kcse past papers with answers - kcse questions on the river and the source - kcse revision notes
kcse biology essay questions and answers pdf kcse biology essays kcse biology essays pdf kcse biology notes kcse biology paper 1 kcse biology paper 1 2017 kcse biology paper 1 2017 pdf kcse biology paper 2 2012 kcse biology paper 2 2015 kcse biology paper 2 2017 kcse biology paper 3 2016 kcse biology paper 3 past papers kcse biology past papers kcse biology past papers and answers kcse biology practical 2016 kcse biology practical past papers kcse biology practicals kcse biology questions and answers kcse biology questions and answers - kcse past papers biology - kcse biology essay questions and answers - kcse chemistry past papers - download kcse past papers with answers - k.c.s.e papers 2015 - k.c.s.e papers 2016 - kcse biology paper 1 2015 - kcse past papers 2015 - kcse past papers 2011 - kcse past papers 2016 - kcse past papers 2017 - 2017 kcse prediction questions - 2018 kcse prediction questions
kcse business paper 1 2016 kcse business past papers kcse business studies past papers kcse chemistry paper 1 2016 kcse chemistry paper 1 2017 kcse chemistry paper 3 2012 kcse chemistry past papers kcse chemistry past papers and answers kcse chemistry practical kcse computer studies paper 1 kcse computer studies paper 2 kcse computer studies paper 2 pdf kcse cre 2016 kcse cre paper 1 2013 kcse cre paper 1 2015 kcse cre paper 1 2016 kcse cre paper 1 2017 kcse cre paper 2 kcse cre paper 2 2016 kcse cre past papers kcse cre past papers and answers kcse english paper 3 2016 kcse english paper 3 2017 kcse essay questions in betrayal in the city kcse exam papers 2018 kcse exam papers answers kcse french paper 1 kcse french paper 2 kcse french past papers kcse general science syllabus kcse geography paper 2 2016 kcse history paper 1 2012 kcse history paper 2 2016 kcse history paper 2 2017 kcse kiswahili paper 1 2017 kcse marking scheme 2016 kcse marking schemes kcse marking schemes 2017 kcse marking schemes pdf kcse mathematics marking schemes kcse mathematics paper 1 2015 kcse mathematics paper 1 2016 kcse mathematics paper 2 2016 kcse mathematics past papers kcse mathematics past papers pdf kcse mock exams kcse mock papers 2015 kcse mock papers 2017 kcse mock papers 2018 kcse mock papers pdf kcse mock papers pdf 2018 kcse mocks 2017 kcse mocks 2018 kcse music past papers kcse online past papers kcse papers 2015 kcse past papers kcse past papers - kcpe and answers - free mocks online - kcse answers past exams question papers - downloads | kcse papers and marking schemes | exams - kcse mathematics paper 1 questions and answers - kcse cre paper 1 questions and answers - knec past papers free downloads - kcse online registration - kcpe - kcse past papers - knec - knec portal - knec past papers for colleges - kasneb - past papers - kasneb past papers for colleges - cpa past papers - https://www.knec.ac.ke/ - www.knec-portal.ac.ke/ - knec portal: kcse results, online registration, kcse result slip. knec portal confirmation - knec portal kcse results - knec examiners portal - knec website kcse past papers - kcpe and answers - free mocks online - kcse answers past exams question papers - downloads | kcse papers and marking schemes | exams - kcse mathematics paper 1 questions and answers - kcse cre paper 1 questions and answers
kcse past papers 2007 kcse past papers 2009 kcse past papers 2010 kcse past papers 2011 kcse past papers 2011 pdf kcse past papers 2012 kcse past papers 2013 kcse past papers 2013 -knec kcse past papers 2014 kcse past papers 2014 pdf kcse past papers 2015 kcse past papers 2015 marking schemes kcse past papers 2015 pdf kcse past papers 2016 kcse past papers 2016 pdf kcse past papers 2017 kcse past papers 2017 pdf kcse past papers agriculture and answers kcse past papers arabic and answers kcse past papers art and design and answers kcse past papers biology kcse past papers building and construction and answers kcse past papers business studies and answers kcse past papers chemistry kcse past papers chemistry and answers kcse past papers chemistry pdf kcse past papers computer studies and answers kcse past papers cre and answers kcse past papers electricity and answers kcse past papers english and answers kcse past papers french and answers kcse past papers general science and answers kcse past papers geography and answers kcse past papers german and answers kcse past papers history and government and answers kcse past papers home science and answers kcse past papers hre and answers kcse past papers ire and answers kcse past papers kenya sign language and answers kcse past papers kiswahili and answers kcse past papers marking scheme kcse past papers maths kcse past papers metal work and answers kcse past papers music and answers kcse past papers pdf download kcse past papers physics and answers kcse past papers physics with answers kcse past papers power mechanics and answers kcse past papers with answers kcse past papers woodwork and answers kcse physics past papers kcse prediction 2017 kcse prediction 2018 kcse prediction 2018 pdf kcse prediction papers 2018 kcse prediction questions 2018 kcse prediction questions and answers kcse questions and answers kcse questions and answers. download free kcse past papers from knec. all marking schemes - questions and answers are sourced from knec. kcse revision kcse revision papers 2014 kcse revision | secondary school | text books | text book centre kcse trial 2017 kcse trial exams 2017 kenyaplex kcse past papers kenyaplex past papers for secondary kiswahili paper 3 questions and answers klb biology form 3 pdf klb cre form 1 klb cre form 3 knec ict past papers knec past papers for colleges knec past papers free download knec past papers pdf knec revision papers knec technical exams past papers kusoma.com past papers maths kcse 2017 mock past papers 2017 mock past papers with answers mokasa mock 2017 page navigation papacambridge computer science igcse past kcse papers past papers in kenya pre mocks 2018 pte knec past papers revision sample essays on betrayal in the city school biology notes school geography notes school physics notes school river and the source themes used in betrayal in the city xtremepapers igcse computer science z notes computer science igcse
Scholarship 2026/27
Current Scholarships 2026/2027 - Fully Funded
Full Undergraduate Scholarships 2026 - 2027
Fully Funded Masters Scholarships 2026 - 27
PhD Scholarships for International Students - Fully Funded!
Funding Opportunities for Journalists 2026/2027
Funding for Entrepreneurs 2026/2027
***
