KCSE Past Papers 2021 Chemistry paper 1(233/1)
Chemistry - Paper 1 2021 - 2 Hours
2021 Chemistry paper 1
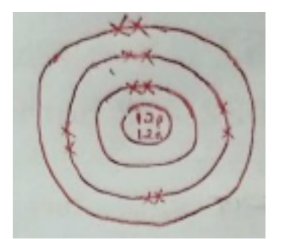
1.a. Draw a labelled diagram showing the atomic structure of 24 12 mg. (2 marks)
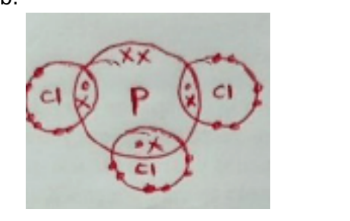
b. The atomic number of phosphorus is 15. Draw a dot (•) and cross (x) diagram for the compound formed when phosphorus reacts with chlorine, atomic number 17. (1 mark)
2.a. State the condition under which a Bunsen burner produces a luminous flame. (1 mark)
b. Write an equation for the reaction that takes place in a luminous flame assuming the laboratory gas is butane. (1 mark)
c. One of the regions in the non-luminous flame is the unburnt gas region. Describe how the presence of this region can be shown using a wooden splint. (1 mark)
3.a. The elements sodium, magnesium and aluminium belong to group I, II and III respectively.Select the element with the highest electrical conductivity and give a reason. (1 mark)
b. Complete Table 1 to show the products of electrolysis for concentrated sodium chloride and molten sodium chloride.
4. A small piece of sodium metal was placed in a beaker containing pure water revision.
a. State two observations made during the reaction. (1 mark)
b. State and explain another observation made when a drop of phenolphthalein is added to the mixture in the beaker. (1 mark)
c. Explain why it is not advisable to carry out this experiment using potassium metal. (1 mark)
5. Describe how a pure sample of copper(II) nitrate crystals can be prepared using recycled copper wire. (3 marks)
6. The following apparatus and chemicals are used to investigate the percentage of air used when iron rusts: iron filings, 100 ml measuring cylinder, trough and water.
a. Draw a setup of the experiment. (2 marks)
b. Write an expression to show how the percentage of air used is calculated at the end of the experiment (1 mark)
7. Figure I shows a graph of atomic radius of some group I and group II elements.
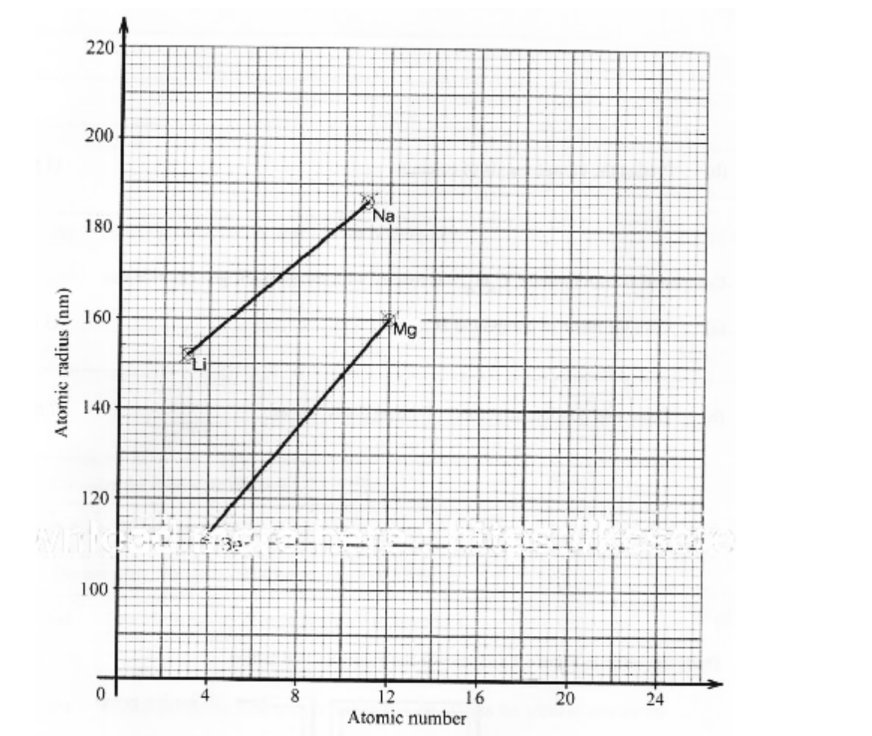
i. lithium (1 mark)
ii. magnesium (1 mark)
b. Predict the atomic radius of calcium. (1 mark)
8. Compound D with formula, C3H4 was reacted with excess hydrogen chloride gas.
a. Give the name of compound D. (1 mark)
b. Draw two possible structures of the products formed. (2 marks)
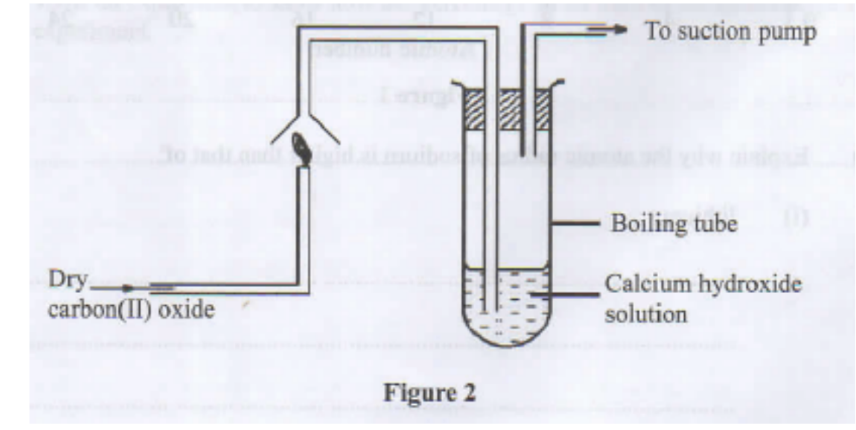
9. Study the setup in Figure 2 and answer the questions that follow.
a. State the precaution that should be taken in carrying out the experiment. Give a reason. (1 mark)
b. State the observations made in the boiling tube. (2 marks)
10. Consider the following reaction:
N2(g) + 3H2(g) ⇌ 2NH3(g)
The enthalpy change is 92.4 kJ per mole of nitrogen.
a. Give the enthalpy change per mole of ammonia. (1 mark)
b. State and explain how each of the following affects the yield of ammonia:
i. Increase in temperature. (1 mark)
ii. Finely divided iron. (1 mark)
11. Study the flow chart in Figure 3 and answer the questions that follow.
a. Identify solid E. (1 mark)
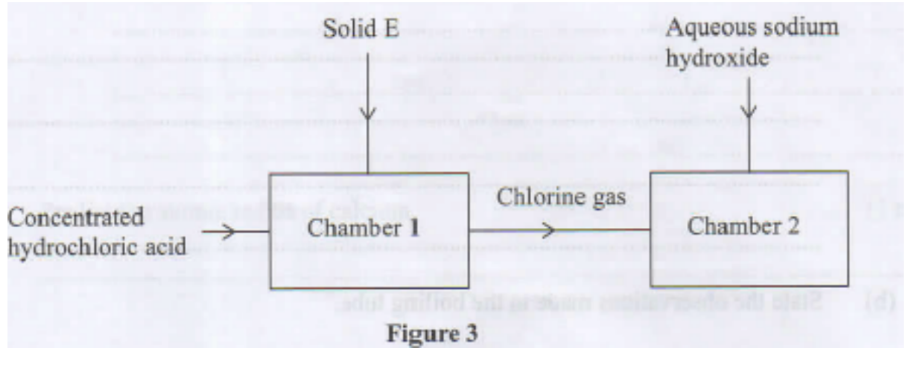
c. Write an equation for the reaction that takes place in chamber 2. (1 mark)
12. Compounds H and J have the following structures.
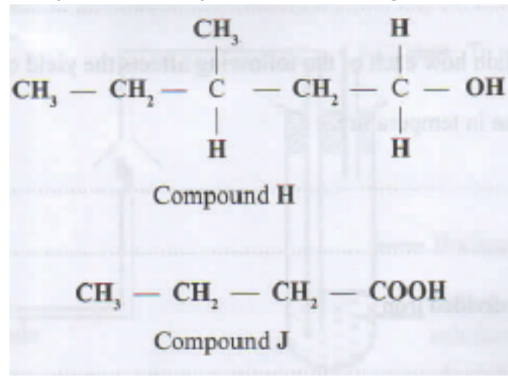
i. Compound H. (1 mark)
ii. Compound J. (1 mark)
b. State the conditions necessary for H and J to react. (1 mark)
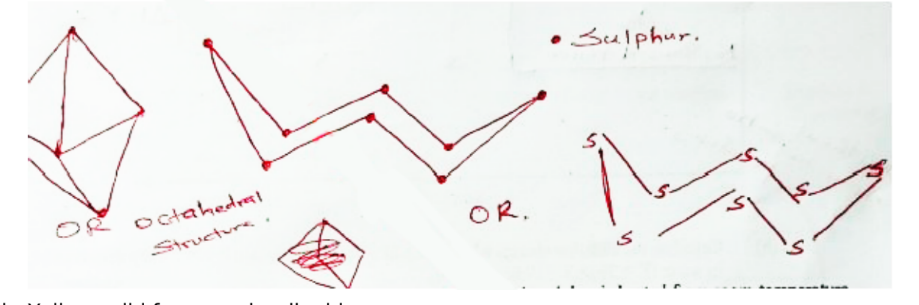
13. Rhombic sulphur is one of the allotropes of sulphur
a. Draw the structure of rhombic sulphur. (1 mark)
b. Describe the observations made when rhombic sulphur is heated from room temperature until it boils. (1 mark)
14. The molar enthalpy of solution for potassium sulphate (K,SO) is +23.8 kJ.
a. On the axes provided, draw a labelled energy level diagram for the dissolution process of potassium sulphate in water. (2 marks)

15.a. State Gay-Lussac's law. (1 mark)
b. 180 cm3 of nitrogen(II) oxide gas was reacted with 400 cm3 of oxygen gas.
i. Write an equation for the reaction. (1 mark)
ii. Calculate the total volume of the gases at the end of the reaction. (3 marks)
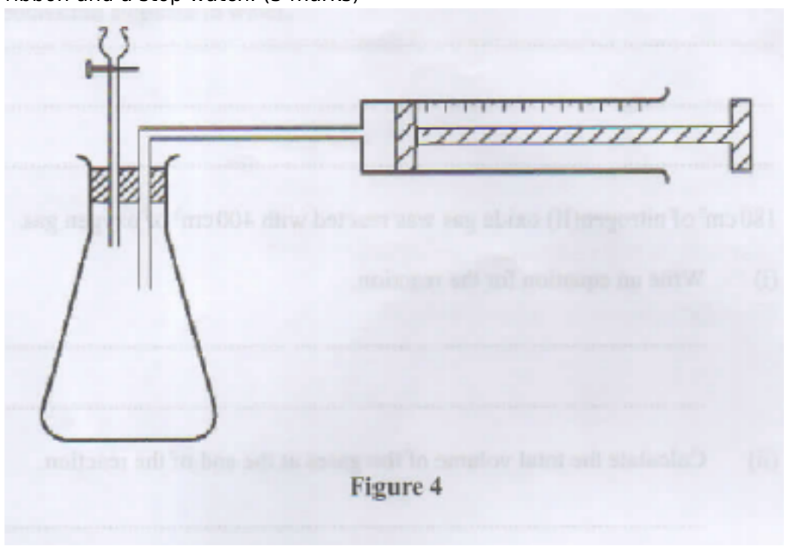
16. Describe how the setup in Figure 4 can be used to distinguish between 50.0 cm of 0.2M hydrochloric acid and 50.0 cm of 0.2 M ethanoic acid using pieces of 6 m length of magnesium ribbon and a stop watch. (3 marks)
samples of sodium carbonate and sodium sulphite. (3 marks)
18.a. Describe how propanone can be used to extract a pure sample of sunflower oil. (2 marks)
b. State why sodium hydroxide solution is not suitable for the extraction of sunflower oil. (1 mark)
19. 31.5 cm3 of concentrated nitric(V) acid was diluted to 500 cm3. 10.0 cm3 of the dilute acid required 25.0 cm3 of 0.4M sodium hydroxide for neutralisation.
a. Calculate concentration of the:
i. dilute acid. (1 mark)
ii. concentrated acid. (1 mark)
20. Figure 5 shows part of a radioactive decay series.
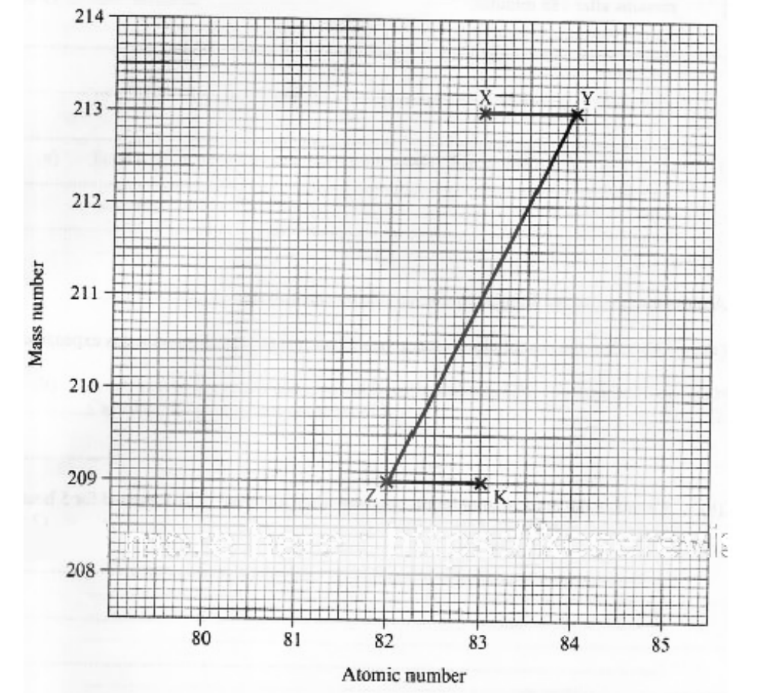
b. The half-life of nuclide X is 47 minutes. Determine the percentage of nuclide X that remains after 188 minutes. (2 marks)
21. Aluminium is extracted from aluminium oxide by electrolysis.
a. Other than the cost of electricity, give another reason why this method is expensive. (1 mark)
b. Calculate the mass of aluminium obtained when a current of 20A is used for 5 hours. (1 Faraday - 96500 C; Al - 27.0)(2 marks)
22. Explain each of the following observations:
a. Articles made of copper turn green when left exposed in air over a long period of time. (1 mark)
b. Addition of aqueous ammonia to a solution containing copper(II)ions produces a deep blue solution (1 mark)
23.a. State what is meant by relative atomic mass of an element. (1 mark)
b.
| Let RAM 7 x be n | C | X | |
|---|---|---|---|
| RAM | 12 | N | 96.4 = 4 n 0.3 |
| % mass | 3.6 12 |
96.4 n |
n = 96.4 1.2 |
| 96.4 n |
|||
| Ratio | 1 | 4 | = 80.3 |
Calculate the relative atomic mass of X. (2 marks)
24. Carbon(II) oxide can be prepared by dehydration of ethanedioic acid
a. Complete the following equation to show the reaction that takes place. (1 mark) H20204
b. Name another reagent that can be used to prepare carbon(II) oxide by dehydration (1 mark)
25. Figure 6 shows an incomplete diagram of a setup for laboratory preparation of nitrogen gas.
b. The nitrogen prepared using this setup is purer than that obtained from air. Give a reason(1 mark)
26. Hydrazine, is used as a fuel in rockets. Using the bond energies in Table 2, calculate the enthalpy
change for combustion of hydrazine.
N₂H4 (1) + O₂(g) → N₂(g) + 2H₂O(g)
Table 2
| Bond | Bond Energy kJ/mol |
|---|---|
| N-H | 388 |
| N-N | 163 |
| O=O | 496 |
| N≡N | 944 |
| O-H | 463 |
State and explain the reactions that take place when aqueous bromine is added to a sample of sea water containing both chloride and iodide ions. (2 marks)
| Reduction equations | Eo/V |
|---|---|
| CI2 + 2e→2CI- | +1.36 |
| Br₂ +2e→2Br- | +1.07 |
| I2 + 2e→21- | +0.54 |
Questions and Answers
Kenya certificate of Secondary Education
2021 Chemistry paper 1
1.a. Draw a labelled diagram showing the atomic structure of 24 12 mg. (2 marks)
b. Write an equation for the reaction that takes place in a luminous flame assuming the laboratory gas is butane. (1 mark)
b. CH4(g) + 4O2(g) → C(s) + 3CO(g) + CO(g) +5H2O(I)
OR
CH4(g) + 4O2(g) → C(s) + CO(g) + CO(g) +5H2O(I)
c. One of the regions in the non-luminous flame is the unburnt gas region. Describe how the presence of this region can be shown using a wooden splint. (1 mark)
3.a. The elements sodium, magnesium and aluminium belong to group I, II and III respectively.
Select the element with the highest electrical conductivity and give a reason. (1 mark)
When airhole/collar is closed or fully closed
b. Complete Table 1 to show the products of electrolysis for concentrated sodium chloride and molten sodium chloride.
CH4(g) + 4O2(g) → C(s) + 3CO(g) + CO(g) +5H2O(I)
OR
CH4(g) + 4O2(g) → C(s) + CO(g) + CO(g) +5H2O(I)
4. A small piece of sodium metal was placed in a beaker containing pure water revision.
a. State two observations made during the reaction. (1 mark)
b. State and explain another observation made when a drop of phenolphthalein is added to the mixture in the beaker. (1 mark)
c. Explain why it is not advisable to carry out this experiment using potassium metal. (1 mark)
5. Describe how a pure sample of copper(II) nitrate crystals can be prepared using recycled copper wire. (3 marks)
6. The following apparatus and chemicals are used to investigate the percentage of air used when iron rusts: iron filings, 100 ml measuring cylinder, trough and water.
a. Draw a setup of the experiment. (2 marks)
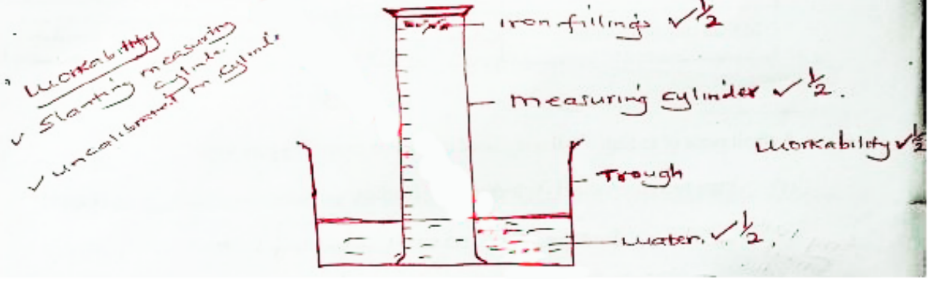
Initial height of air column - Final height of air column/Initial height of air column
OR
Initial height of water - Final height of water/Initial height of water
7. Figure I shows a graph of atomic radius of some group I and group II elements.
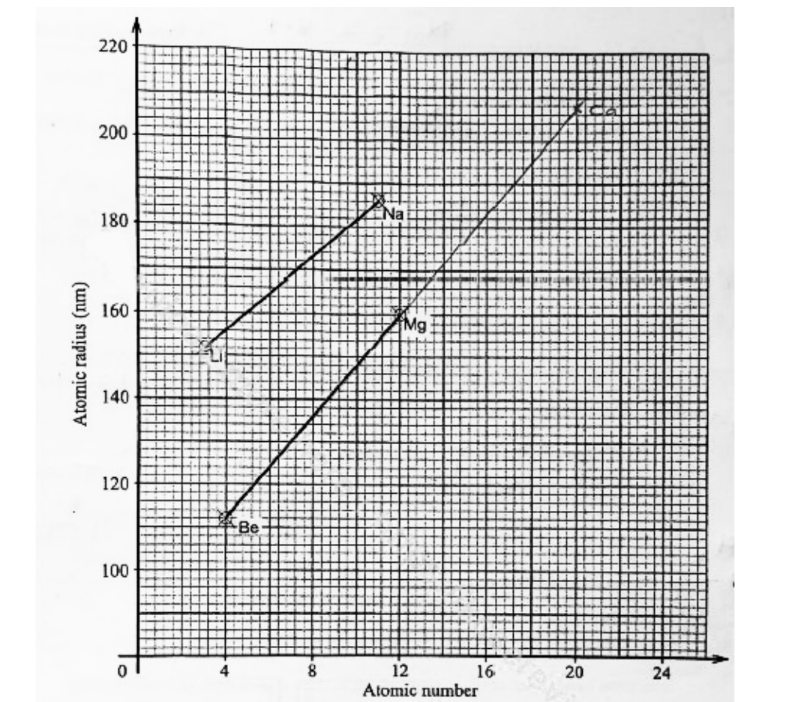
i. lithium (1 mark)
Na = 2.8.1
Li = 2.1
Sodium has 3 energy levels while lithium has two
or
Li = 2
Na = 2.8.1
ii. magnesium (1 mark)
Mg = 2.8.2
Na = 2.8.1
The effective nuclear charge is higher in magnesium than sodium. Mg has a higher number of protons
b. Predict the atomic radius of calcium. (1 mark)
8. Compound D with formula, C3H4 was reacted with excess hydrogen chloride gas.
a. Give the name of compound D. (1 mark)
b. Draw two possible structures of the products formed. (2 marks)
9. Study the setup in Figure 2 and answer the questions that follow.
a. State the precaution that should be taken in carrying out the experiment. Give a reason. (1 mark)
b. State the observations made in the boiling tube. (2 marks)
N2(g) + 3H2(g) ⇌ 2NH3(g)
The enthalpy change is 92.4 kJ per mole of nitrogen.
a. Give the enthalpy change per mole of ammonia. (1 mark)
i. Increase in temperature. (1 mark)
ii. Finely divided iron. (1 mark)
ii. No effect
11. Study the flow chart in Figure 3 and answer the questions that follow.
a. Identify solid E. (1 mark)

c. Write an equation for the reaction that takes place in chamber 2. (1 mark)
12. Compounds H and J have the following structures.

i. Compound H. (1 mark)
ii. Compound J. (1 mark)
b. State the conditions necessary for H and J to react. (1 mark)
a. Draw the structure of rhombic sulphur. (1 mark)
14. The molar enthalpy of solution for potassium sulphate (K,SO) is +23.8 kJ.
a. On the axes provided, draw a labelled energy level diagram for the dissolution process of potassium sulphate in water. (2 marks)
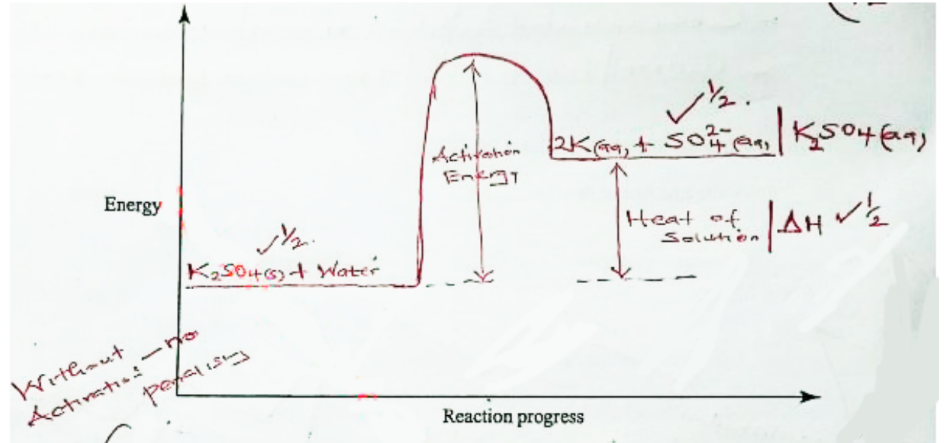
RFM of K2SO2 = 174
moles of K2SO2 =5.22/174 = 0.03
ΔH = 0.03 x 23.8 = 0.714KJ
15.a. State Gay-Lussac's law. (1 mark)
b. 180 cm3 of nitrogen(II) oxide gas was reacted with 400 cm3 of oxygen gas.
i. Write an equation for the reaction. (1 mark)
ii. Calculate the total volume of the gases at the end of the reaction. (3 marks)
using ratio
Volume of oxygen =180 x 1/2 = 90cm3
Volume of oxygen unreacted = 400 - 90
= 310
Volume of NO2 = 18cm3
Total volume = 310 + 180
= 490cm3
16. Describe how the setup in Figure 4 can be used to distinguish between 50.0 cm of 0.2M hydrochloric acid and 50.0 cm of 0.2 M ethanoic acid using pieces of 6 m length of magnesium ribbon and a stop watch. (3 marks)

b. State why sodium hydroxide solution is not suitable for the extraction of sunflower oil. (1 mark)
19. 31.5 cm3 of concentrated nitric(V) acid was diluted to 500 cm3. 10.0 cm3 of the dilute acid required 25.0 cm3 of 0.4M sodium hydroxide for neutralisation.
a. Calculate concentration of the:
i. dilute acid. (1 mark)
Moles of NaOH = 0.4 x 25 = 0.01/1000
Moles of HNO3 = 0.01
Molarity of HNO3 = 0.01 x 1000/10
ii. concentrated acid. (1 mark)
C1V1 = C2V2 1 x 500/31.5 = 15.9M
20. Figure 5 shows part of a radioactive decay series.

Q = It
= 20 x 5 x 60 = 360000
moles = 360000 = 1.244moles
3 x 96500
mass = 1.244 x 27
= 33.588g
b. The half-life of nuclide X is 47 minutes. Determine the percentage of nuclide X that remains after 188 minutes. (2 marks)
21. Aluminium is extracted from aluminium oxide by electrolysis.
a. Other than the cost of electricity, give another reason why this method is expensive. (1 mark)
b. Calculate the mass of aluminium obtained when a current of 20A is used for 5 hours. (1 Faraday - 96500 C; Al - 27.0)(2 marks)
22. Explain each of the following observations:
a. Articles made of copper turn green when left exposed in air over a long period of time. (1 mark)
23.a. State what is meant by relative atomic mass of an element. (1 mark)
b.
| Let RAM 7 x be n | C | X | |
|---|---|---|---|
| RAM | 12 | N | 96.4 = 4 n 0.3 |
| % mass | 3.6 12 |
96.4 n |
n = 96.4 1.2 |
| 96.4 n |
|||
| Ratio | 1 | 4 | = 80.3 |
Calculate the relative atomic mass of X. (2 marks)
24. Carbon(II) oxide can be prepared by dehydration of ethanedioic acid
a. Complete the following equation to show the reaction that takes place. (1 mark) H20204
H2C2O4 → CO(g) + CO2(g) + H2O(I)
b. Name another reagent that can be used to prepare carbon(II) oxide by dehydration (1 mark)
25. Figure 6 shows an incomplete diagram of a setup for laboratory preparation of nitrogen gas.

b. The nitrogen prepared using this setup is purer than that obtained from air. Give a reason(1 mark)
26. Hydrazine, is used as a fuel in rockets. Using the bond energies in Table 2, calculate the enthalpy change for combustion of hydrazine.
N₂H4 (1) + O₂(g) → N₂(g) + 2H₂O(g)
Table 2
| Bond | Bond Energy kJ/mol |
|---|---|
| N-H | 388 |
| N-N | 163 |
| O=O | 496 |
| N≡N | 944 |
| O-H | 463 |
Bonds broken
4 x 388 = 1552
1 x 163 = 163
1 x 496 = 496
= 2211
Bonds formed
1 x 944 = 944
2 x 463 = 1852
= -2796
Enthalpy of combination = -2796 + 2211
= 585KJmol-
27.a. Table 3 gives the standard reduction potentials of some group VII elements. Table 3
State and explain the reactions that take place when aqueous bromine is added to a sample of sea water containing both chloride and iodide ions. (2 marks)
Br2(aq) + 2CI- → No reaction
| Reduction equations | Eo/V |
|---|---|
| CI2 + 2e→2CI- | +1.36 |
| Br₂ +2e→2Br- | +1.07 |
| I2 + 2e→21- | +0.54 |
Secondary School Scholarships in Kenya » Kenya Postgraduate Scholarships » Undergraduate Scholarships for Kenyan Students » Kenya Scholarships for Kenyan Students Studying in Kenya » Kenya Undergraduate Scholarships » The Kenya Youth Education Scholarship Fund - Scholarships Kenya - Scholarships KCSE Results » KCSE Results Top 100 Schools - Kenya Certificate of Secondary Education – KCSE » KCSE Top 100 Candidates » Kenya Certificate of Secondary Education – KCSE » KNEC - Kenya National Examinations Council » Secondary Schools in Kenya » KNEC - Kenya National Examinations Council » Free KNEC KCSE Past Papers
Kenya Scholarships for Undergraduate Students » Kenya Scholarships for Postgraduate Students » Undergraduate Scholarships for Kenyan Students » Kenya Undergraduate Scholarships » Full Undergraduate Scholarships for Kenyans » Kenya Postgraduate Scholarships » Scholarships & Grants » Undergraduate Scholarships » Universities in Kenya » Kenya Universities and Colleges Central Placement Service (KUCCPS) » Colleges in Kenya » KASNEB Registration & Results » Secondary Schools Scholarships in Kenya » Undergraduate & Graduate Scholarships for Kenyans
Powerful Motivational Quotes for Students » Success Quotes for Students » KCSE Motivational Quotes for KCSE Candidates » KCSE Success Quotes for KCSE Candidates
1 a a kcse past papers 2014 kcse marking schemes 2016 kcse papers 2016 kcse prediction questions 2018 kcse exam 2018 kcse questions a a kcse past papers advance-africa.com kcse rev quiz agriculture mock papers agriculture paper 2 questions and answers pdf alliance mocks 2017 ap biology essay questions and answers arabic exam 2016 arabic oral exam questions betrayal in the city essay questions and answers pdf betrayal in the city essay questions with answers betrayal in the city, ,,revision questions biology book 3 klb biology essay questions and answers form 4 biology essay questions and answers form 4 pdf biology essays pdf biology exam questions and answers pdf biology form 2 questions and answers pdf biology form 3 notes pdf biology form 3 questions and answers pdf biology form 3 syllabus biology form three reproduction biology form three-questions and answers biology kcse - kcse biology questions and answers - kcse biology essay questions and answers - kcse biology paper 1 2015 - kcse biology notes - kcse 2015 biology paper 2 - kcse biology practical 2015 - kcse biology practicals - kcse biology 2011
biology kcse 2017 biology kcse questions biology paper 1 questions and answers biology paper 2 questions and answers biology paper 3 questions and answers biology questions and answers for high schools biology questions and answers for high schools pdf biology questions and answers form 2 biology questions and answers multiple choice biology questions and answers on cells biology questions and answers online biology questions and answers pdf biology revision notes form 3 business past kcse past papers c.r.e form one notes pdf cambridge igcse computer science cambridge igcse computer science answers cambridge igcse computer science coursebook pdf download cambridge igcse computer science revision guide pdf cambridge igcse computer science study and revision guide pdf cambridge igcse computer science workbook - free download cambridge igcse computer science workbook pdf caucasian chalk circle essay questions chemistry paper 1 questions and answers chemistry paper 2 questions and answers chemistry paper 3 question and answer chemistry past papers form 1 chemistry past papers form 2 cie past papers computer science 0478 computer science igcse past papers xtremepapers computer science paper 2 2017 computer science past papers a level computer science past papers o level computer studies form 1 questions computer studies form 3 past papers computer studies past papers computer studies questions and answers pdf county mocks 2017 cre form 2 notes pdf cre form 3 notes cre form 3 notes pdf cre form 4 notes cre form 4 notes pdf cre form one notes cre kcse 2016 cre notes cre notes form 2 cre notes pdf cre paper 1 with answers cre paper 2 cre paper 2 topics cre preparation notes cre questions form one cre revision notes cre revision questions and answers download kcse past papers with answers dvance kcse past papers edexcel igcse computer science past papers english paper 3 question paper - 2014 kcse english paper 3 question paper - 2015 kcse english paper 3 question paper - 2016 kcse english paper 3 question paper - 2017 kcse english paper 3 question paper - 2018 kcse essay questions and answers on betrayal in the city essay questions based on betrayal in the city find download kcse past papers with answers - kcse past papers pdf download - kcse 2013 marking scheme - kcse mathematics past papers pdf - free kcse past papers and marking schemes - kcse mock papers pdf - kcse past papers 2014 pdf - kcse past papers 2015 - kcse past papers 2010 find kcse biology essay questions and answers - kcse biology practicals - kcse biology paper 1 2015 - biology essay questions and answers form 4 - kcse biology questions and answers - ap biology essay questions and answers - kcse biology notes - kcse biology paper 2 2012 - kcse biology paper 2 2015
form 2 biology questions and answers free kcse mocks 2015 free kcse past papers - kcse past papers - knec kcse online past papers - knec kcse results past papers free kcse past papers 2014 free kcse past papers kenya, free marking schemes, download ... free kcse past papers with answers free kcse questions and answers on chemistry free revision papers general biology test questions and answers general science questions and answers pdf history and government paper one topics history form one questions and answers pdf history paper 1 questions and answers history paper 2 questions and answers home science past papers igcse computer science book igcse computer science book pdf download igcse computer science notes igcse computer science paper 2 notes igcse computer science past papers igcse computer science past papers 2014 igcse computer science past papers 2017 igcse computer science pdf igcse computer science pre release material 2018 igcse computer science resources igcse computer science revision notes pdf igcse computer science workbook pdf igcse computer studies past papers interesting biology questions ire kcse past papers k.c.s.e cre paper 1 2017 k.c.s.e geography 2017 k.c.s.e mathematics paper 1 2017 k.c.s.e mocks 2018 k.c.s.e past papers 2014 kcpe 2018 predictions kcpe prediction questions kcse 2010 marking scheme kcse 2010 past papers kcse 2011 cre paper 1 kcse 2011 marking scheme kcse 2012 history paper 2 marking scheme kcse 2012 marking schemes kcse 2013 cre paper 1 kcse 2013 marking scheme kcse 2013 marking scheme pdf kcse 2014 kcse 2015 biology paper 2 kcse 2015 biology paper 3 kcse 2015 marking scheme kcse 2015 past papers kcse 2016 agriculture paper 2 kcse 2016 biology paper 1 kcse 2016 biology paper 2 kcse 2016 computer paper 1 kcse 2017 marking scheme kcse 2017 maths paper 1 kcse 2017 papers kcse 2017 papers and marking scheme kcse 2017 past papers kcse 2017 prediction pdf kcse 2018 cre prediction kcse 2018 leakage kcse 2018 marking scheme kcse 2018 papers kcse 2018 predictions kcse 2019 marking scheme kcse agriculture past papers kcse answers kcse arabic paper 1 kcse arabic paper 2 kcse arabic paper 3 kcse arabic paper 3 2016 kcse arabic past papers kcse biology 2011 kcse biology essay questions and answers kcse biology essay questions and answers - kcse revision questions and answers - kcse chemistry questions and answers - kcse revision papers with answers - kcse past papers with answers - download kcse past papers with answers - kcse questions on the river and the source - kcse revision notes
kcse biology essay questions and answers - kcse revision questions and answers - kcse chemistry questions and answers - kcse revision papers with answers - kcse past papers with answers - download kcse past papers with answers - kcse questions on the river and the source - kcse revision notes
kcse biology essay questions and answers pdf kcse biology essays kcse biology essays pdf kcse biology notes kcse biology paper 1 kcse biology paper 1 2017 kcse biology paper 1 2017 pdf kcse biology paper 2 2012 kcse biology paper 2 2015 kcse biology paper 2 2017 kcse biology paper 3 2016 kcse biology paper 3 past papers kcse biology past papers kcse biology past papers and answers kcse biology practical 2016 kcse biology practical past papers kcse biology practicals kcse biology questions and answers kcse biology questions and answers - kcse past papers biology - kcse biology essay questions and answers - kcse chemistry past papers - download kcse past papers with answers - k.c.s.e papers 2015 - k.c.s.e papers 2016 - kcse biology paper 1 2015 - kcse past papers 2015 - kcse past papers 2011 - kcse past papers 2016 - kcse past papers 2017 - 2017 kcse prediction questions - 2018 kcse prediction questions
kcse business paper 1 2016 kcse business past papers kcse business studies past papers kcse chemistry paper 1 2016 kcse chemistry paper 1 2017 kcse chemistry paper 3 2012 kcse chemistry past papers kcse chemistry past papers and answers kcse chemistry practical kcse computer studies paper 1 kcse computer studies paper 2 kcse computer studies paper 2 pdf kcse cre 2016 kcse cre paper 1 2013 kcse cre paper 1 2015 kcse cre paper 1 2016 kcse cre paper 1 2017 kcse cre paper 2 kcse cre paper 2 2016 kcse cre past papers kcse cre past papers and answers kcse english paper 3 2016 kcse english paper 3 2017 kcse essay questions in betrayal in the city kcse exam papers 2018 kcse exam papers answers kcse french paper 1 kcse french paper 2 kcse french past papers kcse general science syllabus kcse geography paper 2 2016 kcse history paper 1 2012 kcse history paper 2 2016 kcse history paper 2 2017 kcse kiswahili paper 1 2017 kcse marking scheme 2016 kcse marking schemes kcse marking schemes 2017 kcse marking schemes pdf kcse mathematics marking schemes kcse mathematics paper 1 2015 kcse mathematics paper 1 2016 kcse mathematics paper 2 2016 kcse mathematics past papers kcse mathematics past papers pdf kcse mock exams kcse mock papers 2015 kcse mock papers 2017 kcse mock papers 2018 kcse mock papers pdf kcse mock papers pdf 2018 kcse mocks 2017 kcse mocks 2018 kcse music past papers kcse online past papers kcse papers 2015 kcse past papers kcse past papers - kcpe and answers - free mocks online - kcse answers past exams question papers - downloads | kcse papers and marking schemes | exams - kcse mathematics paper 1 questions and answers - kcse cre paper 1 questions and answers - knec past papers free downloads - kcse online registration - kcpe - kcse past papers - knec - knec portal - knec past papers for colleges - kasneb - past papers - kasneb past papers for colleges - cpa past papers - https://www.knec.ac.ke/ - www.knec-portal.ac.ke/ - knec portal: kcse results, online registration, kcse result slip. knec portal confirmation - knec portal kcse results - knec examiners portal - knec website kcse past papers - kcpe and answers - free mocks online - kcse answers past exams question papers - downloads | kcse papers and marking schemes | exams - kcse mathematics paper 1 questions and answers - kcse cre paper 1 questions and answers
kcse past papers 2007 kcse past papers 2009 kcse past papers 2010 kcse past papers 2011 kcse past papers 2011 pdf kcse past papers 2012 kcse past papers 2013 kcse past papers 2013 -knec kcse past papers 2014 kcse past papers 2014 pdf kcse past papers 2015 kcse past papers 2015 marking schemes kcse past papers 2015 pdf kcse past papers 2016 kcse past papers 2016 pdf kcse past papers 2017 kcse past papers 2017 pdf kcse past papers agriculture and answers kcse past papers arabic and answers kcse past papers art and design and answers kcse past papers biology kcse past papers building and construction and answers kcse past papers business studies and answers kcse past papers chemistry kcse past papers chemistry and answers kcse past papers chemistry pdf kcse past papers computer studies and answers kcse past papers cre and answers kcse past papers electricity and answers kcse past papers english and answers kcse past papers french and answers kcse past papers general science and answers kcse past papers geography and answers kcse past papers german and answers kcse past papers history and government and answers kcse past papers home science and answers kcse past papers hre and answers kcse past papers ire and answers kcse past papers kenya sign language and answers kcse past papers kiswahili and answers kcse past papers marking scheme kcse past papers maths kcse past papers metal work and answers kcse past papers music and answers kcse past papers pdf download kcse past papers physics and answers kcse past papers physics with answers kcse past papers power mechanics and answers kcse past papers with answers kcse past papers woodwork and answers kcse physics past papers kcse prediction 2017 kcse prediction 2018 kcse prediction 2018 pdf kcse prediction papers 2018 kcse prediction questions 2018 kcse prediction questions and answers kcse questions and answers kcse questions and answers. download free kcse past papers from knec. all marking schemes - questions and answers are sourced from knec. kcse revision kcse revision papers 2014 kcse revision | secondary school | text books | text book centre kcse trial 2017 kcse trial exams 2017 kenyaplex kcse past papers kenyaplex past papers for secondary kiswahili paper 3 questions and answers klb biology form 3 pdf klb cre form 1 klb cre form 3 knec ict past papers knec past papers for colleges knec past papers free download knec past papers pdf knec revision papers knec technical exams past papers kusoma.com past papers maths kcse 2017 mock past papers 2017 mock past papers with answers mokasa mock 2017 page navigation papacambridge computer science igcse past kcse papers past papers in kenya pre mocks 2018 pte knec past papers revision sample essays on betrayal in the city school biology notes school geography notes school physics notes school river and the source themes used in betrayal in the city xtremepapers igcse computer science z notes computer science igcse
Scholarship 2026/27
Current Scholarships 2026/2027 - Fully Funded
Full Undergraduate Scholarships 2026 - 2027
Fully Funded Masters Scholarships 2026 - 27
PhD Scholarships for International Students - Fully Funded!
Funding Opportunities for Journalists 2026/2027
Funding for Entrepreneurs 2026/2027
***
