KCSE Past Papers 2017 Chemistry Paper 1
2017 Chemistry paper 1
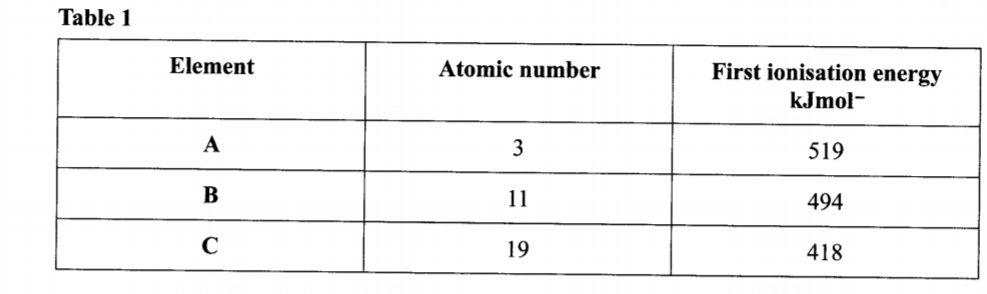
1. Table 1 shows the atomic numbers and the first ionisation energies of three elements. The letters are not actual symbols of the elements. Use it to answer the questions that follow.
(b) Write the electronic configuration for the ion of C. (1 mark)
2. Calculate the values of X and Y in the following nuclear equation. 239U_> X Th+2 oc+2/3 92 Y (2 mark)
3. The diagram in Figure 1 shows a section of a dry cell. Study it and answer the questions that follow.
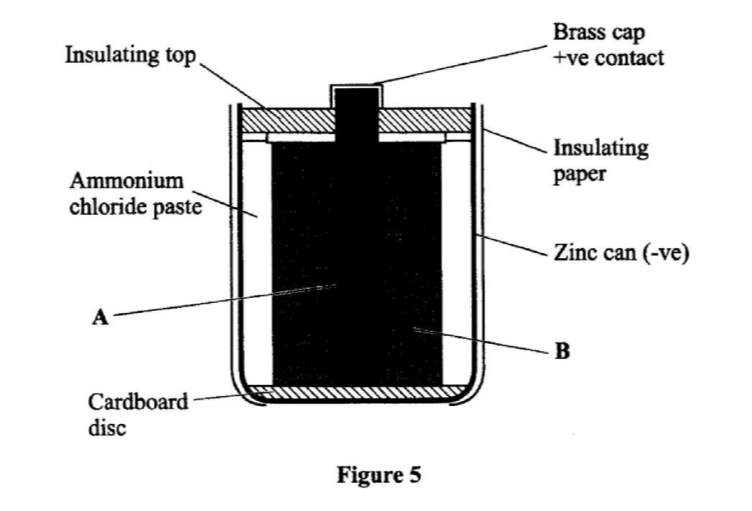
(b) The part labelled A is a paste. Give a reason why it is not used in dry form. (1 mark)
(c) What is the purpose of the zinc container? (1 mark)
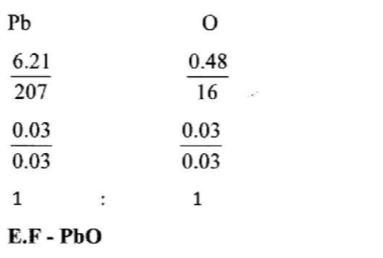
4. The empirical formula of lead(II) oxide was determined by passing excess dry hydrogen gas over 6.69g of heated lead(II) oxide.
(a) What was the purpose of using excess dry hydrogen gas? (2 marks)
(b) The mass of lead was found to he 6.21g.
Determine the empirical formula of the oxide. (Pb = 207.0 0 = 16.0) (2 marks)
5. The set-up in Figure 2 was used to prepare a sample of ethane gas. Study it and answer the questions that follow.
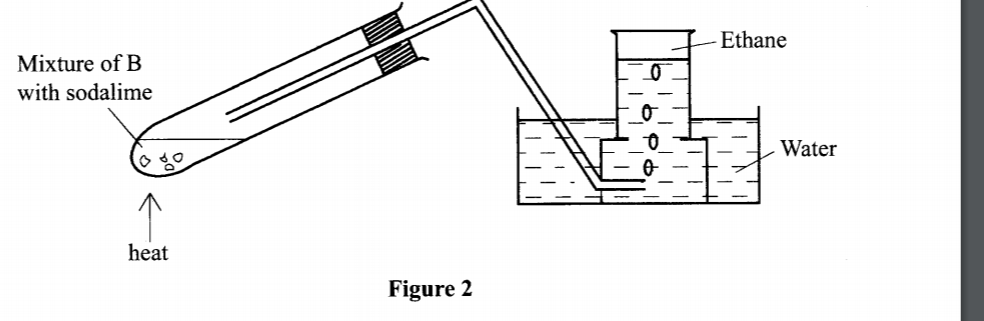
(b) Explain why the pressure of a fixed mass of a gas increases, when the volume of the gas is reduced at constant temperature. (2 marks)
7. A sample of water is suspected to contain sulphate ions. Describe an experiment that can be carried out to determine the presence of sulphate ions. (3 marks)
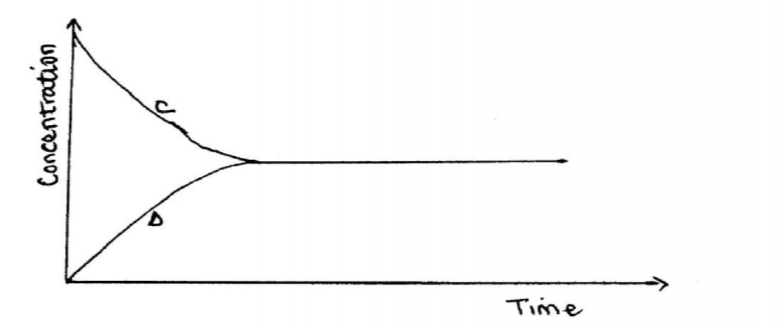
8. (a) State one characteristic of a reaction where equilibrium has been attained. (1 mark)
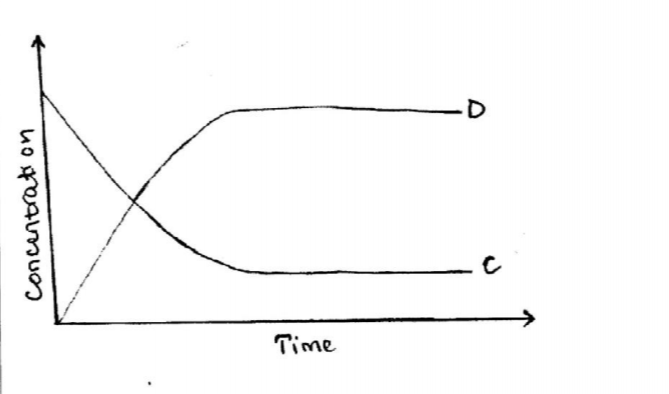
(b) The following equation is in a state of equilibrium: C D Use it to sketch a graphical representation of concentration against time in seconds for the equilibrium. (2 marks)
9. Copper(II) ions react with excess aqueous ammonia to form a complex ion.
(a) (i) Write an equation for the reaction that forms the complex ion. ( I mark)
(ii) Name the complex ion. ( I mark)
(b) Explain why CH4 is not acidic while HCl is acidic yet both compounds contain hydrogen. (1 mark)
10. 20 cm3 of ethanoic acid was diluted to 400 cm3 of solution. Calculate the concentration of the solution in moles per litre. (C = 12.0 ; H = 1.0 ; 0 =16.0) (Density of ethanoic acid = 1.05 g/cm3) (3 marks)
11. An oxide of element K has the formula 1(205. (a) Determine the oxidation number of K. (1 mark)
(b) To which group of the periodic table does K belong? (1 mark)
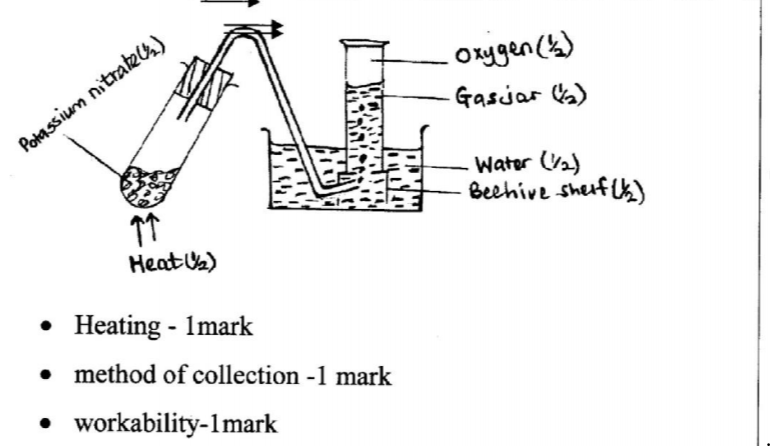
12. Potassium nitrate liberates oxygen gas when heated. Draw a diagram of a set-up that shows heating of potassium nitrate and collection of oxygen gas. (3 marks)
13. Explain the observation made when chlorine gas is passed through a solution of potassium iodide. (3 marks)
14. Using the elements chlorine, calcium and phosphorus:
(a) Select elements that will form an oxide whose aqueous solution has a pH less than 7. ( I mark)
(b) Write an equation for the reaction between calcium oxide and dilute hydrochloric acid. (1 mark)
(c) Give one use of calcium oxide. ( I mark)
15. Starting with copper, describe how a pure sample of copper(II) carbonate can be prepared. (3 marks)
16. In an experiment, concentrated nitric(V) acid was reacted with iron(II) sulphate. State and explain the observations made. (2 marks)
17. The flow chart in Figure 3 shows the process of obtaining a sample of nitrogen gas. Study it and answer the questions that follow.
(b) Write an equation for the reaction with heated copper turnings. (1 mark)
(c) Name an impurity in the sample of nitrogen gas. ( I mark)
18. The set-up in Figure 4 can be used to prepare nitrogen(II) oxide. Use it to answer the questions that follow.
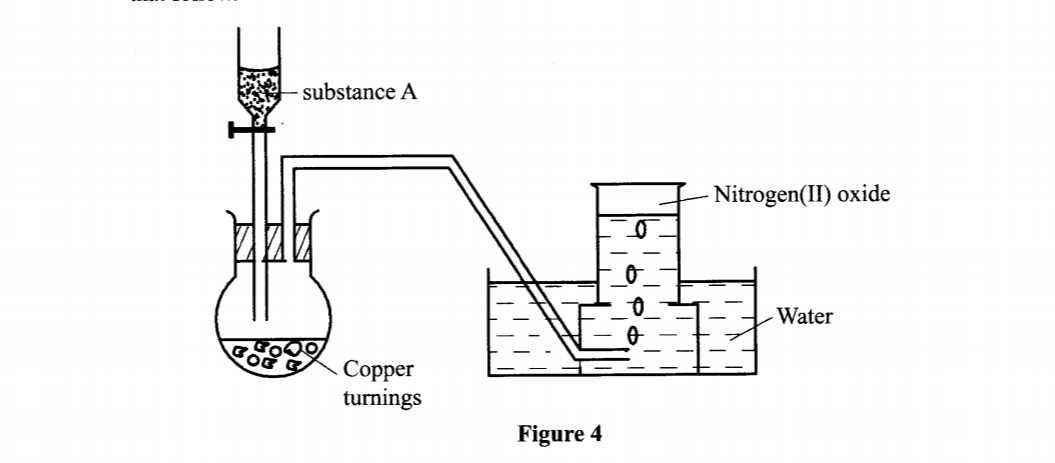
(c) Write an equation for the reaction which occurred in the flask. (1 mark)
19. The following procedure was used to investigate the temperature changes that occur when sodium hydroxide solution is added to dilute hydrochloric acid.
(i) Place the acid in a glass beaker and record its temperature.
(ii) Add a known volume of sodium hydroxide solution.
(iii) Stir the mixture and record the highest temperature reached.
(iv) Repeat steps
(ii) and
(iii) with different volumes of sodium hydroxide solution.
(a) State two factors that must be kept constant in this experiment (1 mark)
(b) Explain how the use of a polystyrene cup will affect the results. (1 mark)
20. Study the flow chart in Figure 5 and answer the questions that follow.
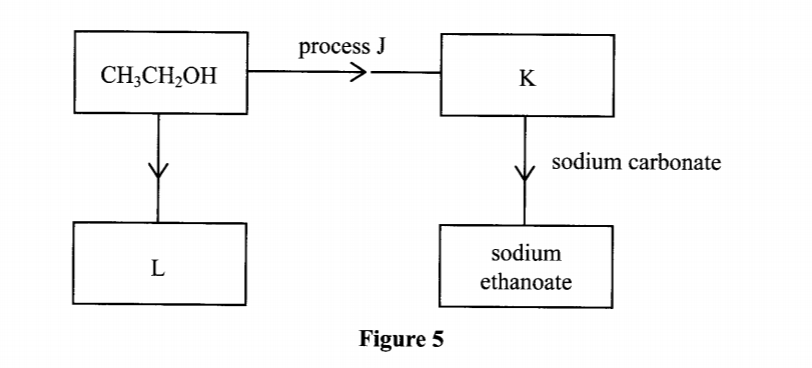
(b) Name one reagent that can be used to carry out process J. (1 mark)
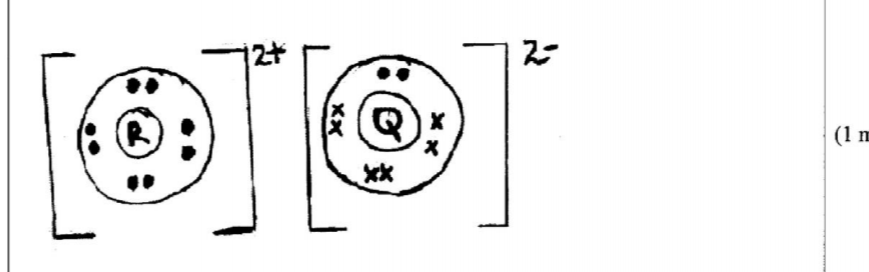
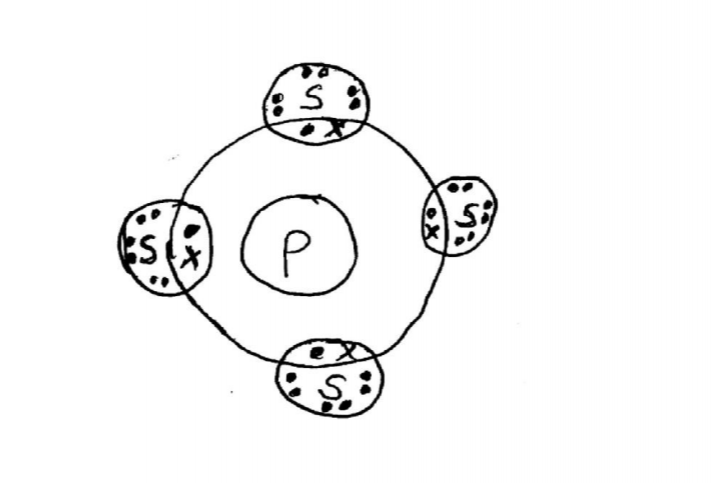
21. The atomic numbers of some elements P, Q, R and S are 6, 8, 12 and 17 respectively.
(a) Draw the dot (•) and cross (X) diagrams for the compounds formed when:
(i) R and Q react (1 mark)
(ii) P and S react. (1 mark)
(b) Explain why the melting point of the compound formed by P and S is lower than that formed by R and Q. (1 mark)
22. (a) What is an inert electrode? (1 mark)
(b) State the products formed when brine is electrolysed using inert electrodes. Anode: (1 mark)
Cathode: (1 mark)
23. Explain how a student can establish whether a liquid sample extracted from a plant is pure. (2 marks)
Anode (1 mark)
Cathode (1 mark)
23. Explain how a student can establish whether a liquid sample extracted from a plant is pure. (2 marks)
24. Figure 6 shows part of the periodic table. The letters are not the actual symbols of the elements. Stud it and answer the questions that follow.
(b) Select the element which can form an ion with a charge of +3. (1 mark)
(c) An element J has atomic number 15. Indicate with a tick (✓), on the part of the periodic table the position of J. (1 mark)
25. In terms of structure and bonding, explain why graphite is used as a lubricant in machines. (3 marks)
26. (a) What is meant by the term bleaching? (1 mark)
(b) Write the formula of the bleaching nent formed when chlorine gas reacts with aqueous sodium hydroxide. y ( 1 mark)
(c) State the role of chlorine in water treatment. (1 mark)
27. (a) Name two ores in which sodium occurs. ( 1 mark)
(b) During extraction of sodium using the down's process, calcium chloride is added to the ore. Give a reason for the addition of calcium chloride. (1 mark)
(c) State two uses of sodium. ( I mark)
28. When an aqueous solution of compound X was mixed with a few drops of bromine water, the colour of the mixture remained yellow.
When another portion of solution X was reacted with acidified potassium dichromate(VI), the colour of the mixture changed from orange to green.
(a) What conclusion can be made from the use of:
(i) bromine water? (1 mark)
(ii) acidified potassium dichromate(VI)? (1 mark)
(b) Solution X was reacted with a piece of a metal and a colourless gas was produced. Describe a simple experiment to identify the gas. (1 mark)
Questions and Answers
2017 Chemistry paper 1
1. Table 1 shows the atomic numbers and the first ionisation energies of three elements. The letters are not actual symbols of the elements. Use it to answer the questions that follow.

- Ionisation energy decreases down the group 1 elements.
- This is because atomic radii increases from A to C (down the group) /outermost electron is far from nucleus hence requires less energy to be lost during reaction.
(b) Write the electronic configuration for the ion of C. (1 mark)
- Electron configuration of ion of C- 2.8.8
2. Calculate the values of X and Y in the following nuclear equation. 239U_> X Th+2 oc+2/3 92 Y (2 mark)
- x = 231
- y = 90
3. The diagram in Figure 1 shows a section of a dry cell. Study it and answer the questions that follow.

- Carbon electrode (Anode) / Graphite electrode
(b) The part labelled A is a paste. Give a reason why it is not used in dry form. (1 mark)
- To allow movement of ions / to have it as an electrolyte. When dry, the ions are immobile.
(c) What is the purpose of the zinc container? (1 mark)
- It is the cathode / negative electrode
4. The empirical formula of lead(II) oxide was determined by passing excess dry hydrogen gas over 6.69g of heated lead(II) oxide.
(a) What was the purpose of using excess dry hydrogen gas? (2 marks)
- To ensure all the oxide was reduced.
(b) The mass of lead was found to he 6.21g. Determine the empirical formula of the oxide. (Pb = 207.0 0 = 16.0) (2 marks)
- Mass of oxygen 6.69 — 6.21
5. The set-up in Figure 2 was used to prepare a sample of ethane gas. Study it and answer the questions that follow.

(b) Write an equation for the complete combustion of ethane. (1 mark)
2C2H6(g) + 702(g) -> 4CO
(c) State one use of ethane. (1 mark) - as Fuel - Production of ethene - Production of hydrogen gas
6. (a) State Charles' Law. (1 mark)
- The volume of a fixed mass of a gas is directly proportional to the absolute temperature at constant pressure.
(b) Explain why the pressure of a fixed mass of a gas increases, when the volume of the gas is reduced at constant temperature. (2 marks) - As the volume decreases, there is increased bombardment / collisions of the molecules against the walls of the container, hence increased pressure.
7. A sample of water is suspected to contain sulphate ions. Describe an experiment that can be carried out to determine the presence of sulphate ions. (3 marks)
• Add aqueous barium nitrate / barium chloride to sample; Followed by dilute nitric(V) acid or HCI; • If white precipitate persists, then S042- ionsare present; • If the precipitate dissolves then S042- ionsare absent.
OR
• Add lead(II) nitrate solution
8. (a) State one characteristic of a reaction where equilibrium has been attained. (1 mark) - The concentrations of reactants and products remain constant or Rate of forward reactions is equal to the rate of backward reaction.
(b) The following equation is in a state of equilibrium: C D Use it to sketch a graphical representation of concentration against time in seconds for the equilibrium. (2 marks)
(a) (i) Write an equation for the reaction that forms the complex ion. ( I mark)
Cu(OH)2(s)+ 4NH3 (aq) -+ [Cu(NH 3)4]2++ (aq)+2OH-- (aq)
OR Cu2+ (act), 4NH3 (aq) [Cu(NH3)4]2+(aq)
(ii) Name the complex ion. ( I mark)
- Tetraamine copper(II)ion
(b) Explain why CH4 is not acidic while HCl is acidic yet both compounds contain hydrogen. (1 mark) - CH4 is a hydrocarbon, non-polar hence does not ionize in water. HCI is polar hence ionizes in water.
10. 20 cm3 of ethanoic acid was diluted to 400 cm3 of solution. Calculate the concentration of the solution in moles per litre. (C = 12.0 ; H = 1.0 ; 0 =16.0) (Density of ethanoic acid = 1.05 g/cm3) (3 marks)
Molar mass of ethanoic acid (CH3COOH) = 60g
Mass of ethanoic acid = 20 x 1.05g/cm3 =21g 21 60
Moles of ethanoic = 0.35 moles
Molarity = 0.35 400/1000 = 0.875M
11. An oxide of element K has the formula 1(205. (a) Determine the oxidation number of K. (1 mark) = 2k+(5x-2)=0
2k=+10
k=+5
(b) To which group of the periodic table does K belong? (1 mark)
- Group 5
12. Potassium nitrate liberates oxygen gas when heated. Draw a diagram of a set-up that shows heating of potassium nitrate and collection of oxygen gas. (3 marks) - A dark grey / brown solid is deposited / the solution turns black; chlorine is more reactive / a stronger oxidizing agent than iodine; Therefore displaces it from a solution of its ions OR
C12 (g) + 21- (aq) 2 Cr-(aq) + 12 (S)
14. Using the elements chlorine, calcium and phosphorus:
(a) Select elements that will form an oxide whose aqueous solution has a pH less than 7. ( I mark) - Phosphorus and chlorine
(b) Write an equation for the reaction between calcium oxide and dilute hydrochloric acid. (1 mark) - CaO(,) + 2HCI(,q) --> CaCl2(aq) + H2O)
(c) Give one use of calcium oxide. ( I mark) - used to neutralize acidic soil / liming;
- drying vent;
15. Starting with copper, describe how a pure sample of copper(II) carbonate can be prepared. (3 marks)
- To copper turnings, add 50% concentration H2SO4 or HNO3 / Heat copper turnings to form copper(II) oxide and add dilute H2SO4 or HNO3 or HC1; To the resulting mixture, add excess sodium carbonate (soluble) Filter mixture; Rinse residue with water and dry between filter papers.
16. In an experiment, concentrated nitric(V) acid was reacted with iron(II) sulphate. State and explain the observations made. (2 marks) - The mixture changed from green to yellow / formation of a brown gas; Iron(II) ions is oxidized by nitric(V) acid to Iron(III) ions / nitric(V) acid is reduced to nitrogen(II) oxide which is oxidized by oxygen to nitrogen(IV) ) oxide.
17. The flow chart in Figure 3 shows the process of obtaining a sample of nitrogen gas. Study it and answer the questions that follow.
- Sodium hydroxide solution or Potassium hydroxide solution;
(b) Write an equation for the reaction with heated copper turnings.
(1 mark) - 12 Cu(s) + 02(g ) -> 2 CuO (s)
(c) Name an impurity in the sample of nitrogen gas. ( I mark) - Argon, - Neon, - Inert gases
18. The set-up in Figure 4 can be used to prepare nitrogen(II) oxide. Use it to answer the questions that follow.
- Moderately concentration nitric(V) acid / 50% concentrated nitric(V) acid.
(c) Write an equation for the reaction which occurred in the flask. (1 mark)
3Cu (s) + 8HNO3 (aq) 3Cu (NO3)2 (aq)+ 4H2O +2NO
19. The following procedure was used to investigate the temperature changes that occur when sodium hydroxide solution is added to dilute hydrochloric acid.
(i) Place the acid in a glass beaker and record its temperature.
(ii) Add a known volume of sodium hydroxide solution. (iii) Stir the mixture and record the highest temperature reached. (iv) Repeat steps (ii) and (iii) with different volumes of sodium hydroxide solution.
(a) State two factors that must be kept constant in this experiment (1 mark)
- concentration of acid and base
- Volume of acid used.
(b) Explain how the use of a polystyrene cup will affect the results. (1 mark)
- Improves accuracy; - Polystyrene is a plastic and will not absorb heat /minimum heat loss
20. Study the flow chart in Figure 5 and answer the questions that follow.
K:- Ethanoic acid / (CH3COOH) L:- Ethene (1 mark) (b) Name one reagent that can be used to carry out process J. (1 mark) - Acidified potassium dichromate(VI) OR acidified potassium manganate(VII)
21. The atomic numbers of some elements P, Q, R and S are 6, 8, 12 and 17 respectively. (a) Draw the dot (•) and cross (X) diagrams for the compounds formed when: (i) R and Q react (1 mark) - Rand Q form an ionic compound with strong ionic bonds while R and S form a covalent compound seith weak Van der Waals forces.
22. (a) What is an inert electrode? (1 mark)
- is one which does not participate in the reaction / does not affect the products of electrolysis / does not react; Anode - chlorine; Cathode - Hydrogen
(b) State the products formed when brine is electrolysed using inert electrodes.
Anode: (1 mark) - Chlorine
Cathode: (1 mark) - Hydrogen
23. Explain how a student can establish whether a liquid sample extracted from a plant is pure. (2 marks) - Measure the boiling point / freezing point; -If the boiling point /freezing point is sharp, then liquid is pure.
24. Figure 6 shows part of the periodic table. The letters are not the actual symbols of the elements. Stud it and answer the questions that follow.
4M(s) +K,(g) —> 2M2K(S) OR 4K(s) + 02(g) 2K20(s)
(b) Select the element which can form an ion with a charge of +3. (1 mark)
- J should be placed in period 3, group 5 of the periodic table
(c) An element J has atomic number 15. Indicate with a tick (✓), on the part of the periodic table the position of J. (1 mark)
25. In terms of structure and bonding, explain why graphite is used as a lubricant in machines. (3 marks)
- Graphite consists of layers of carbon atoms; - The layers are held together by the weak Van der Waals forces of attraction; - These layers therefore slide over each other thus preventing machine to machine contact.
26. (a) What is meant by the term bleaching? (1 mark)
- Removal of original colour from a substance and the remaining substance is white / colourless
(b) Write the formula of the bleaching agent formed when chlorine gas reacts with aqueous sodium hydroxide. ( 1 mark) - NaCIO / NaOCI
(c) State the role of chlorine in water treatment. (1 mark)
- Kill germs / bacteria / microorganisms
27. (a) Name two ores in which sodium occurs. ( 1 mark)
• rock salt /NaCI / trona ; • salt petre/ NaNO3.
(b) During extraction of sodium using the down's process, calcium chloride is added to the ore. Give a reason for the addition of calcium chloride. (1 mark)
To lower the melting point from 800°C to about 600°C;
(c) State two uses of sodium. ( I mark) • street lighting; • coolant in nuclear reactors; • extraction of titanium; • extraction of gold; • manufacture of sodium cyanide; • manufacture of sodium peroxide.
28. When an aqueous solution of compound X was mixed with a few drops of bromine water, the colour of the mixture remained yellow. When another portion of solution X was reacted with acidified potassium dichromate(VI), the colour of the mixture changed from orange to green.
(a) What conclusion can be made from the use of:
(i) bromine water? (1 mark)
(ii) acidified potassium dichromate(VI)? (1 mark)
— OH / R — OH present
(b) Solution X was reacted with a piece of a metal and a colourless gas was produced. Describe a simple experiment to identify the gas. (1 mark)
Lower a burning splint to the gas, a 'pop' sound should be produced showing it is hydrogen.
Secondary School Scholarships in Kenya » Kenya Postgraduate Scholarships » Undergraduate Scholarships for Kenyan Students » Kenya Scholarships for Kenyan Students Studying in Kenya » Kenya Undergraduate Scholarships » The Kenya Youth Education Scholarship Fund - Scholarships Kenya - Scholarships
KCSE Results » KCSE Results Top 100 Schools - Kenya Certificate of Secondary Education – KCSE » KCSE Top 100 Candidates » Kenya Certificate of Secondary Education – KCSE » KNEC - Kenya National Examinations Council » Secondary Schools in Kenya » KNEC - Kenya National Examinations Council » Free KNEC KCSE Past Papers
Kenya Scholarships for Undergraduate Students » Kenya Scholarships for Postgraduate Students » Undergraduate Scholarships for Kenyan Students » Kenya Undergraduate Scholarships » Full Undergraduate Scholarships for Kenyans » Kenya Postgraduate Scholarships » Scholarships & Grants » Undergraduate Scholarships » Universities in Kenya » Kenya Universities and Colleges Central Placement Service (KUCCPS) » Colleges in Kenya » KASNEB Registration & Results » Secondary Schools Scholarships in Kenya » Undergraduate & Graduate Scholarships for Kenyans
Powerful Motivational Quotes for Students » Success Quotes for Students » KCSE Motivational Quotes for KCSE Candidates » KCSE Success Quotes for KCSE Candidates
1 a a kcse past papers
2014 kcse marking schemes
2016 kcse papers
2016 kcse prediction questions
2018 kcse exam
2018 kcse questions
a a kcse past papers
advance-africa.com kcse rev quiz
agriculture mock papers
agriculture paper 2 questions and answers pdf
alliance mocks 2017
ap biology essay questions and answers
arabic exam 2016
arabic oral exam questions
betrayal in the city essay questions and answers pdf
betrayal in the city essay questions with answers
betrayal in the city, ,,revision questions
biology book 3 klb
biology essay questions and answers form 4
biology essay questions and answers form 4 pdf
biology essays pdf
biology exam questions and answers pdf
biology form 2 questions and answers pdf
biology form 3 notes pdf
biology form 3 questions and answers pdf
biology form 3 syllabus
biology form three reproduction
biology form three-questions and answers
biology kcse - kcse biology questions and answers - kcse biology essay questions and answers - kcse biology paper 1 2015 - kcse biology notes - kcse 2015 biology paper 2 - kcse biology practical 2015 - kcse biology practicals - kcse biology 2011
biology kcse 2017
biology kcse questions
biology paper 1 questions and answers
biology paper 2 questions and answers
biology paper 3 questions and answers
biology questions and answers for high schools
biology questions and answers for high schools pdf
biology questions and answers form 2
biology questions and answers multiple choice
biology questions and answers on cells
biology questions and answers online
biology questions and answers pdf
biology revision notes form 3
business past kcse past papers
c.r.e form one notes pdf
cambridge igcse computer science
cambridge igcse computer science answers
cambridge igcse computer science coursebook pdf download
cambridge igcse computer science revision guide pdf
cambridge igcse computer science study and revision guide pdf
cambridge igcse computer science workbook - free download
cambridge igcse computer science workbook pdf
caucasian chalk circle essay questions
chemistry paper 1 questions and answers
chemistry paper 2 questions and answers
chemistry paper 3 question and answer
chemistry past papers form 1
chemistry past papers form 2
cie past papers
computer science 0478
computer science igcse past papers xtremepapers
computer science paper 2 2017
computer science past papers a level
computer science past papers o level
computer studies form 1 questions
computer studies form 3 past papers
computer studies past papers
computer studies questions and answers pdf
county mocks 2017
cre form 2 notes pdf
cre form 3 notes
cre form 3 notes pdf
cre form 4 notes
cre form 4 notes pdf
cre form one notes
cre kcse 2016
cre notes
cre notes form 2
cre notes pdf
cre paper 1 with answers
cre paper 2
cre paper 2 topics
cre preparation notes
cre questions form one
cre revision notes
cre revision questions and answers
download kcse past papers with answers
dvance kcse past papers
edexcel igcse computer science past papers
english paper 3 question paper - 2014 kcse
english paper 3 question paper - 2015 kcse
english paper 3 question paper - 2016 kcse
english paper 3 question paper - 2017 kcse
english paper 3 question paper - 2018 kcse
essay questions and answers on betrayal in the city
essay questions based on betrayal in the city
find download kcse past papers with answers - kcse past papers pdf download - kcse 2013 marking scheme - kcse mathematics past papers pdf - free kcse past papers and marking schemes - kcse mock papers pdf - kcse past papers 2014 pdf - kcse past papers 2015 - kcse past papers 2010
find kcse biology essay questions and answers - kcse biology practicals - kcse biology paper 1 2015 - biology essay questions and answers form 4 - kcse biology questions and answers - ap biology essay questions and answers - kcse biology notes - kcse biology paper 2 2012 - kcse biology paper 2 2015
form 2 biology questions and answers
free kcse mocks 2015
free kcse past papers - kcse past papers - knec kcse online past papers - knec kcse results past papers
free kcse past papers 2014
free kcse past papers kenya, free marking schemes, download ...
free kcse past papers with answers
free kcse questions and answers on chemistry
free revision papers
general biology test questions and answers
general science questions and answers pdf
history and government paper one topics
history form one questions and answers pdf
history paper 1 questions and answers
history paper 2 questions and answers
home science past papers
igcse computer science book
igcse computer science book pdf download
igcse computer science notes
igcse computer science paper 2 notes
igcse computer science past papers
igcse computer science past papers 2014
igcse computer science past papers 2017
igcse computer science pdf
igcse computer science pre release material 2018
igcse computer science resources
igcse computer science revision notes pdf
igcse computer science workbook pdf
igcse computer studies past papers
interesting biology questions
ire kcse past papers
k.c.s.e cre paper 1 2017
k.c.s.e geography 2017
k.c.s.e mathematics paper 1 2017
k.c.s.e mocks 2018
k.c.s.e past papers 2014
kcpe 2018 predictions
kcpe prediction questions
kcse 2010 marking scheme
kcse 2010 past papers
kcse 2011 cre paper 1
kcse 2011 marking scheme
kcse 2012 history paper 2 marking scheme
kcse 2012 marking schemes
kcse 2013 cre paper 1
kcse 2013 marking scheme
kcse 2013 marking scheme pdf
kcse 2014
kcse 2015 biology paper 2
kcse 2015 biology paper 3
kcse 2015 marking scheme
kcse 2015 past papers
kcse 2016 agriculture paper 2
kcse 2016 biology paper 1
kcse 2016 biology paper 2
kcse 2016 computer paper 1
kcse 2017 marking scheme
kcse 2017 maths paper 1
kcse 2017 papers
kcse 2017 papers and marking scheme
kcse 2017 past papers
kcse 2017 prediction pdf
kcse 2018 cre prediction
kcse 2018 leakage
kcse 2018 marking scheme
kcse 2018 papers
kcse 2018 predictions
kcse 2019 marking scheme
kcse agriculture past papers
kcse answers
kcse arabic paper 1
kcse arabic paper 2
kcse arabic paper 3
kcse arabic paper 3 2016
kcse arabic past papers
kcse biology 2011
kcse biology essay questions and answers
kcse biology essay questions and answers - kcse revision questions and answers - kcse chemistry questions and answers - kcse revision papers with answers - kcse past papers with answers - download kcse past papers with answers - kcse questions on the river and the source - kcse revision notes
kcse biology essay questions and answers - kcse revision questions and answers - kcse chemistry questions and answers - kcse revision papers with answers - kcse past papers with answers - download kcse past papers with answers - kcse questions on the river and the source - kcse revision notes
kcse biology essay questions and answers pdf
kcse biology essays
kcse biology essays pdf
kcse biology notes
kcse biology paper 1
kcse biology paper 1 2017
kcse biology paper 1 2017 pdf
kcse biology paper 2 2012
kcse biology paper 2 2015
kcse biology paper 2 2017
kcse biology paper 3 2016
kcse biology paper 3 past papers
kcse biology past papers
kcse biology past papers and answers
kcse biology practical 2016
kcse biology practical past papers
kcse biology practicals
kcse biology questions and answers
kcse biology questions and answers - kcse past papers biology - kcse biology essay questions and answers - kcse chemistry past papers - download kcse past papers with answers - k.c.s.e papers 2015 - k.c.s.e papers 2016 - kcse biology paper 1 2015 - kcse past papers 2015 - kcse past papers 2011 - kcse past papers 2016 - kcse past papers 2017 - 2017 kcse prediction questions - 2018 kcse prediction questions
kcse business paper 1 2016
kcse business past papers
kcse business studies past papers
kcse chemistry paper 1 2016
kcse chemistry paper 1 2017
kcse chemistry paper 3 2012
kcse chemistry past papers
kcse chemistry past papers and answers
kcse chemistry practical
kcse computer studies paper 1
kcse computer studies paper 2
kcse computer studies paper 2 pdf
kcse cre 2016
kcse cre paper 1 2013
kcse cre paper 1 2015
kcse cre paper 1 2016
kcse cre paper 1 2017
kcse cre paper 2
kcse cre paper 2 2016
kcse cre past papers
kcse cre past papers and answers
kcse english paper 3 2016
kcse english paper 3 2017
kcse essay questions in betrayal in the city
kcse exam papers 2018
kcse exam papers answers
kcse french paper 1
kcse french paper 2
kcse french past papers
kcse general science syllabus
kcse geography paper 2 2016
kcse history paper 1 2012
kcse history paper 2 2016
kcse history paper 2 2017
kcse kiswahili paper 1 2017
kcse marking scheme 2016
kcse marking schemes
kcse marking schemes 2017
kcse marking schemes pdf
kcse mathematics marking schemes
kcse mathematics paper 1 2015
kcse mathematics paper 1 2016
kcse mathematics paper 2 2016
kcse mathematics past papers
kcse mathematics past papers pdf
kcse mock exams
kcse mock papers 2015
kcse mock papers 2017
kcse mock papers 2018
kcse mock papers pdf
kcse mock papers pdf 2018
kcse mocks 2017
kcse mocks 2018
kcse music past papers
kcse online past papers
kcse papers 2015
kcse past papers
kcse past papers - kcpe and answers - free mocks online - kcse answers past exams question papers - downloads | kcse papers and marking schemes | exams - kcse mathematics paper 1 questions and answers - kcse cre paper 1 questions and answers - knec past papers free downloads - kcse online registration - kcpe - kcse past papers - knec - knec portal - knec past papers for colleges - kasneb - past papers - kasneb past papers for colleges - cpa past papers - https://www.knec.ac.ke/ - www.knec-portal.ac.ke/ - knec portal: kcse results, online registration, kcse result slip. knec portal confirmation - knec portal kcse results - knec examiners portal - knec website
kcse past papers - kcpe and answers - free mocks online - kcse answers past exams question papers - downloads | kcse papers and marking schemes | exams - kcse mathematics paper 1 questions and answers - kcse cre paper 1 questions and answers
kcse past papers 2007
kcse past papers 2009
kcse past papers 2010
kcse past papers 2011
kcse past papers 2011 pdf
kcse past papers 2012
kcse past papers 2013
kcse past papers 2013 -knec
kcse past papers 2014
kcse past papers 2014 pdf
kcse past papers 2015
kcse past papers 2015 marking schemes
kcse past papers 2015 pdf
kcse past papers 2016
kcse past papers 2016 pdf
kcse past papers 2017
kcse past papers 2017 pdf
kcse past papers agriculture and answers
kcse past papers arabic and answers
kcse past papers art and design and answers
kcse past papers biology
kcse past papers building and construction and answers
kcse past papers business studies and answers
kcse past papers chemistry
kcse past papers chemistry and answers
kcse past papers chemistry pdf
kcse past papers computer studies and answers
kcse past papers cre and answers
kcse past papers electricity and answers
kcse past papers english and answers
kcse past papers french and answers
kcse past papers general science and answers
kcse past papers geography and answers
kcse past papers german and answers
kcse past papers history and government and answers
kcse past papers home science and answers
kcse past papers hre and answers
kcse past papers ire and answers
kcse past papers kenya sign language and answers
kcse past papers kiswahili and answers
kcse past papers marking scheme
kcse past papers maths
kcse past papers metal work and answers
kcse past papers music and answers
kcse past papers pdf download
kcse past papers physics and answers
kcse past papers physics with answers
kcse past papers power mechanics and answers
kcse past papers with answers
kcse past papers woodwork and answers
kcse physics past papers
kcse prediction 2017
kcse prediction 2018
kcse prediction 2018 pdf
kcse prediction papers 2018
kcse prediction questions 2018
kcse prediction questions and answers
kcse questions and answers
kcse questions and answers. download free kcse past papers from knec. all marking schemes - questions and answers are sourced from knec.
kcse revision
kcse revision papers 2014
kcse revision | secondary school | text books | text book centre
kcse trial 2017
kcse trial exams 2017
kenyaplex kcse past papers
kenyaplex past papers for secondary
kiswahili paper 3 questions and answers
klb biology form 3 pdf
klb cre form 1
klb cre form 3
knec ict past papers
knec past papers for colleges
knec past papers free download
knec past papers pdf
knec revision papers
knec technical exams past papers
kusoma.com past papers
maths kcse 2017
mock past papers 2017
mock past papers with answers
mokasa mock 2017
page navigation
papacambridge computer science igcse
past kcse papers
past papers in kenya
pre mocks 2018
pte knec past papers
revision
sample essays on betrayal in the city
school biology notes
school geography notes
school physics notes
school river and the source
themes used in betrayal in the city
xtremepapers igcse computer science
z notes computer science igcse




KCPE Results » List of National Schools in Kenya (Classified According to Clusters) » National Secondary Schools in Kenya » List of All Secondary Schools in Kenya Per County » Form 1 Intake - Selection Criteria, Selection List » KCSE Results » Secondary Schools in Kenya » KNEC - Kenya National Examinations Council
KCPE Results Performance » KNEC - Kenya National Examinations Council » KCSE Results
KCSE Past Papers 2017 Chemistry Paper 1
"Pdf" Revision Questions Chemistry Form 2
"Pdf" Revision Questions Chemistry Form 3
"Pdf" Revision Questions Chemistry Form 4
"Pdf" Revision Questions Chemistry Form Four
"Pdf" Revision Questions Chemistry Form One
"Pdf" Revision Questions Chemistry Form Three
"Pdf" Revision Questions Chemistry Form Two
1 a a KCSE Past Papers
10th Grade Chemistry Questions and Answers
10th Grade Chemistry Test
11th Ncert Chemistry
12th Class Chemistry Book Free Download
2014 KCSE Marking Schemes
2014 Pdf KCSE Past Papers 2015
2015 Chemistry Essay Questions and Answers Form 4
2016 KCSE Papers
2016 KCSE Prediction Questions
2017 Chemistry Hsc Answers
2017 KCSE Prediction Questions
2018 Chemistry KCSE Leakage
2018 Chemistry KCSE Questions
2018 KCSE Busineness Studies
2018 KCSE Exam
2018 KCSE Leakage
2018 KCSE Prediction Questions
2018 KCSE Questions
2019 Chemistry KCSE Leakage
2019 Chemistry KCSE Questions
2019 KCSE Leakage
2019 KCSE Questions
9th Grade Chemistry Study Guide
A a a Chemistry Notes
a a a Chemistry Notes!
a a a ChemistryNotes!
A a KCSE Past Papers
A Biblical View of Social Justice
A Level Chemistry Biological Molecules Questions
A Level Chemistry Exam Questions by Topic
A Level Chemistry Notes Edexcel
A Level Chemistry Notes Xtremepapers
A Level Chemistry Past Papers
A Level Chemistry Questions and Answers
a Level Chemistry Questions and Answers
A Level Chemistry Questions and Answers (Pdf)
A Level Chemistry Questions and Answers Pdf
A Level Chemistry Questions by Topic Kidney Questions With Markschemes
A Level Chemistry Revision
A Level Chemistry Revision Edexcel
A Level Chemistry Revision Guide
A Level Chemistry Revision Notes
A Level Chemistry Revision Notes Pdf
A Level Chemistry Textbook Pdf
A Level Chemistry Year 1 / as Aqa Exam Questions by Topic
A Level Edexcel Notes a* Chemistry
aa Chemistry Form 3 Questions and Answers
Advance KCSE Past Papers
Advance-africa.com KCSE Rev Quiz
Advantages and Disadvantages.
All Chemistry Essays
All Chemistry Notes for Senior Two
All KCSE Past Papers Chemistry With Making Schemes
All Marking Schemes Questions and Answers
All Past K.c.s.e Questions With Answers
Alliance Mocks 2017
Ap Bio Quizzes
Ap Chemistry 1 Textbook Pdf
Ap Chemistry Essay Questions and Answers
Are Sourced From KNEC.
As Level Chemistry Notes
Atika Chemistry Notes
Atika School Chemistry Notes
B/s Book 2 Notes
Basic Chemistry Books Pdf
basic Chemistry Interview Questions and Answers Pdf
Basic Chemistry Interview Questions and Answers Pdf
Basic Chemistry Pdf
Basic Chemistry Questions and Answers
Basic Chemistry Questions and Answers Pdf
Bbc Bitesize Chemistry Ks3
Bihar Board Chemistry Objective Answer 2017
Bihar Board Chemistry Objective Answer 2018
Bio Answers
Bio Quesions
Chemistry 0478
Chemistry 101
Chemistry 12th
Chemistry 12th Class Notes Pdf
Chemistry 2019 Syllabus
Chemistry All KCSE Short Notes
Chemistry Answers
Chemistry Answers Online Free
Chemistry Answers Quizlet
Chemistry Bk 2 Notes
Chemistry Book 1
Chemistry Book 1 Notes
Chemistry Book 2
Chemistry Book 2 Notes
Chemistry Book 3
Chemistry Book 3 KLB
Chemistry Book 3 Notes
Chemistry Book 4
Chemistry Book 4 Notes
Chemistry Book 4 Pdf
Chemistry Book for Class 11
Chemistry Book Four
Chemistry Book Four Notes
Chemistry Book One
Chemistry Book One Notes
Chemistry Book Pdf Free Download
Chemistry Book Three
Chemistry Book Three Notes
Chemistry Book Three Pdf
Chemistry Book Two
Chemistry Book Two Notes
Chemistry Books Form Three
Chemistry Bowl Chemistry Study Guide
Chemistry Bowl Questions Chemistry
Chemistry Bowl Questions Earth Chemistry
Chemistry Bowl Questions Math
Chemistry Bowl Questions Middle School
Chemistry Brekthrough Form Two Notes
Chemistry Class 12 Ncert Solutions
Chemistry Class 12 Pdf
Chemistry Communication Syllabus
Chemistry Diagram Software
Chemistry Diagrams for Class 11
Chemistry Diagrams for Class 12
Chemistry Diagrams for Class 9
Chemistry Diagrams for Class-10
Chemistry Diagrams in Form 1
Chemistry Diagrams in Form 2
Chemistry Diagrams in Form 3
Chemistry Diagrams in Form 4
Chemistry Diagrams Pdf
Chemistry Diagrams to Label
Chemistry Essay Questions and Answers
Chemistry Essay Questions and Answers 2018
Chemistry Essay Questions and Answers Form 1
Chemistry Essay Questions and Answers Form 2
Chemistry Essay Questions and Answers Form 3
Chemistry Essay Questions and Answers Form 4
Chemistry Essay Questions and Answers Form 4 Pdf
Chemistry Essay Questions and Answers Pdf
Chemistry Essay Revision Q
Chemistry Essays and Answers
Chemistry Essays Form One to Form Four
Chemistry Essays Form One to Form Three
Chemistry Essays KCSE
Chemistry Essays Pdf
Chemistry Exam 1 Multiple Choice
Chemistry Exam 2 Advance
Chemistry Exam 2 Test
Chemistry Exam 2016
Chemistry Exam Form Four
Chemistry Exam Form One
Chemistry Exam Form Three
Chemistry Exam Form Two
Chemistry Exam Practice Test
Chemistry Exam Questions
Chemistry Exam Questions and Answers
Chemistry Exam Questions and Answers Pdf
Chemistry Exam Study Guide
Chemistry Exams
Chemistry Excretion Notes
Chemistry Exercise Form 4 With Answers
Chemistry Final Exam Answer Key
Chemistry Final Exam Answer Key 2016
Chemistry Final Exam Answer Key 2017
Chemistry Final Exam Answers 2018
Chemistry Final Exam Answers 2019
Chemistry Final Exam Questions and Answers
Chemistry Fom 1 Notes
Chemistry Fom 2 Notes
Chemistry Fom 3 Notes
Chemistry Fom 4 Notes
Chemistry Form 1
Chemistry Form 1 & 2 and Answers
Chemistry Form 1 and 2 Essays
Chemistry Form 1 and 2 Essays Questions and Answers
Chemistry Form 1 Chapter 1
Chemistry Form 1 Diagrams
Chemistry Form 1 Exams
Chemistry Form 1 Mid Year Exam
Chemistry Form 1 Notes
Chemistry Form 1 Notes and Questions
Chemistry Form 1 Notes Download
Chemistry Form 1 Notes Free Download
Chemistry Form 1 Notes GCSE
Chemistry Form 1 Notes KCSE-kcse
Chemistry Form 1 Notes Pdf
Chemistry Form 1 Notes Pdf Download
Chemistry Form 1 Past Papers
Chemistry Form 1 Pdf
Chemistry Form 1 Pressure
Chemistry Form 1 Question Papers
Chemistry Form 1 Questions
Chemistry Form 1 Questions and Answers
Chemistry Form 1 Questions and Answers Pdf
Chemistry Form 1 Quiz
Chemistry Form 1 Revision Questions
Chemistry Form 1 Summary Notes
Chemistry Form 1 Syllabus
Chemistry Form 1 Work
Chemistry Form 1-4 Notes
Chemistry Form 2
Chemistry Form 2 Chapter 1
Chemistry Form 2 Chapter 2
Chemistry Form 2 Diagrams
Chemistry Form 2 Exam Paper 2014
Chemistry Form 2 Exams
Chemistry Form 2 Notes
Chemistry Form 2 Notes and Questions
Chemistry Form 2 Notes GCSE
Chemistry Form 2 Notes KCSE-kcse
Chemistry Form 2 Notes Pdf
Chemistry Form 2 Notes Pdf Download
Chemistry Form 2 Past Papers
Chemistry Form 2 Pdf
Chemistry Form 2 Question Papers
Chemistry Form 2 Questions
Chemistry Form 2 Questions and Answers
Chemistry Form 2 Questions and Answers Pdf
Chemistry Form 2 Quiz
Chemistry Form 2 Revision Notes
Chemistry Form 2 Salts
Chemistry Form 2 Structure and Bonding
Chemistry Form 2 Summary Notes
Chemistry Form 2 Syllabus
Chemistry Form 2 Work
Chemistry Form 3
Chemistry Form 3 and 4 Essays
Chemistry Form 3 and 4 Essays Questions and Answers
Chemistry Form 3 Chapter 3
Chemistry Form 3 Classification
Chemistry Form 3 Diagrams
Chemistry Form 3 Ecology
Chemistry Form 3 Exams
Chemistry Form 3 Notes
Chemistry Form 3 Notes and Questions
Chemistry Form 3 Notes GCSE
Chemistry Form 3 Notes KCSE-kcse
Chemistry Form 3 Notes Pdf
Chemistry Form 3 Notes Pdf Download
Chemistry Form 3 Notes Topic 1
Chemistry Form 3 Past Papers
Chemistry Form 3 Pdf
Chemistry Form 3 Question Papers
Chemistry Form 3 Questions
Chemistry Form 3 Questions and Answers
Chemistry Form 3 Questions and Answers Pdf
Chemistry Form 3 Questions and Answers Term 3
Chemistry Form 3 Questions and Answers+pdf
Chemistry Form 3 Quiz
Chemistry Form 3 Revision Notes
Chemistry Form 3 Revision Questions
Chemistry Form 3 Summary Notes
Chemistry Form 3 Syllabus
Chemistry Form 3 Syllabus Pdf
Chemistry Form 3 Topics
Chemistry Form 3 Work
Chemistry Form 4
Chemistry Form 4 All Chapter
Chemistry Form 4 Chapter 1 Conversion of Units
Chemistry Form 4 Chapter 1 Exercise
Chemistry Form 4 Chapter 1 Exercise and Answers
Chemistry Form 4 Chapter 1 Exercise Pdf
Chemistry Form 4 Chapter 1 Mind Map
Chemistry Form 4 Chapter 2
Chemistry Form 4 Chapter 2 Exercise and Answers
Chemistry Form 4 Chapter 2 Exercise Pdf
Chemistry Form 4 Chapter 2 Experiment
Chemistry Form 4 Chapter 2 Formula
Chemistry Form 4 Chapter 2 Mind Map
Chemistry Form 4 Chapter 2 Momentum
Chemistry Form 4 Chapter 2 Notes Pdf
Chemistry Form 4 Chapter 2 Objective Questions and Answers
Chemistry Form 4 Chapter 2 Paper 2
Chemistry Form 4 Chapter 2 Slideshare
Chemistry Form 4 Chapter 3
Chemistry Form 4 Chapter 3 Questions and Answers
Chemistry Form 4 Chapter 4
Chemistry Form 4 Chapter 4 Notes Pdf
Chemistry Form 4 Chapter 5 Light Questions and Answers
Chemistry Form 4 Chapter 5 Notes Pdf
Chemistry Form 4 Diagrams
Chemistry Form 4 Exam Paper 1
Chemistry Form 4 Exams
Chemistry Form 4 Exercise
Chemistry Form 4 Exercise Pdf
Chemistry Form 4 Module With Answer
Chemistry Form 4 Note
Chemistry Form 4 Notes
Chemistry Form 4 Notes (Pdf)
Chemistry Form 4 Notes All Chapter Pdf
Chemistry Form 4 Notes and Questions
Chemistry Form 4 Notes Chapter 1
Chemistry Form 4 Notes Chapter 2
Chemistry Form 4 Notes Chapter 3
Chemistry Form 4 Notes Download
Chemistry Form 4 Notes Free Download
Chemistry Form 4 Notes GCSE
Chemistry Form 4 Notes KCSE-kcse
Chemistry Form 4 Notes Pdf
Chemistry Form 4 Notes Pdf Download
Chemistry Form 4 Paper 2 Questions and Answers
Chemistry Form 4 Past Papers
Chemistry Form 4 Question Papers
Chemistry Form 4 Questions
Chemistry Form 4 Questions and Answers
Chemistry Form 4 Questions and Answers Pdf
Chemistry Form 4 Quiz
Chemistry Form 4 Revision Notes
Chemistry Form 4 Schemes of Work
Chemistry Form 4 Summary Notes
Chemistry Form 4 Syllabus
Chemistry Form 4 Textbook Pdf
Chemistry Form 4 Work
Chemistry Form 5 Chapter 1 Exercise and Answers
Chemistry Form 5 Chapter 1 Notes Pdf
Chemistry Form 5 Chapter 2 Notes Pdf
Chemistry Form 5 Chapter 2 Slideshare
Chemistry Form 5 Chapter 3 Notes Pdf
Chemistry Form 5 Notes Pdf
Chemistry Form Four Book
Chemistry Form Four Notes
Chemistry Form Four Notes and Questions
Chemistry Form Four Notes GCSE
Chemistry Form Four Notes Pdf
Chemistry Form Four Past Papers
Chemistry Form Four Questions
Chemistry Form Four Questions and Answers
Chemistry Form Four Questions and Answers Pdf
Chemistry Form Four Quiz
Chemistry Form Four Study Notes
Chemistry Form Four Syllabus
Chemistry Form Four Topic 2
Chemistry Form Four Topic 4
Chemistry Form Four Topics
Chemistry Form Four Work
Chemistry Form One
Chemistry Form One Book
Chemistry Form One Book Pdf
Chemistry Form One Download Topic 1 Upto 3
Chemistry Form One Exam
Chemistry Form One Notes
Chemistry Form One Notes and Questions
Chemistry Form One Notes GCSE
Chemistry Form One Notes Pdf
Chemistry Form One Pdf
Chemistry Form One Questions
Chemistry Form One Questions and Answers
Chemistry Form One Questions and Answers Pdf
Chemistry Form One Questions and Their Answers
Chemistry Form One Quiz
Chemistry Form One Revision Question
Chemistry Form One Schemes of Work
Chemistry Form One Study Notes
Chemistry Form One Syllabus
Chemistry Form One Term Three Test
Chemistry Form One to Three Notes
Chemistry Form One Work
Chemistry Form Three
Chemistry Form Three Book
Chemistry Form Three Notes
Chemistry Form Three Notes and Questions
Chemistry Form Three Notes GCSE
Chemistry Form Three Questions and Answers
Chemistry Form Three Questions and Answers Pdf
Chemistry Form Three Quiz
Chemistry Form Three Reproduction
Chemistry Form Three Reproduction.
Chemistry Form Three Study Notes
Chemistry Form Three Work
Chemistry Form Three-questions and Answers
Chemistry Form Two
Chemistry Form Two Book
Chemistry Form Two Diagrams
Chemistry Form Two Notes
Chemistry Form Two Notes and Questions
Chemistry Form Two Notes GCSE
Chemistry Form Two Notes Pdf
Chemistry Form Two Notes-pdf
Chemistry Form Two Pdf
Chemistry Form Two Questions
Chemistry Form Two Questions and Answers
Chemistry Form Two Questions and Answers Pdf
Chemistry Form Two Quiz
Chemistry Form Two Study Notes
Chemistry Form Two Topics
Chemistry Form Two Work
Chemistry Form Two,schemes of Work
Chemistry Form2
Chemistry Form2 Textbook
Chemistry Game Form Four Question End Answers
Chemistry Grade 10 Exam Papers
Chemistry Hsc Pdf
Chemistry Human Reproduction Video
Chemistry IGCSE Past Papers Xtremepapers
Chemistry K.c.s.e 2017
Chemistry KCSE
Chemistry KCSE 2016
Chemistry KCSE 2017
Chemistry KCSE 2017 Paper 1
Chemistry KCSE Past Papers
Chemistry KCSE Questions
Chemistry KCSE Questions and Answer
Chemistry KCSE Quizzes & Answers
Chemistry KCSE Revision
Chemistry KCSE Revision Notes
Chemistry KCSE Setting Questions Form One and Two
Chemistry Ksce 2015
Chemistry Last Year K.c.s.e Questions
Chemistry Lesson Plan Form Two
Chemistry Made Familiar
Chemistry Mcq for Class 11
Chemistry Mcq for Class 12
Chemistry Mcq for Competitive Exams
Chemistry Mcq for Competitive Exams Pdf
Chemistry Mcq for Neet Pdf
Chemistry Mcq for Ssc
Chemistry Mcq Questions With Answers
Chemistry Mcq With Answers Pdf
Chemistry Mcqs for Class 12 Pdf
Chemistry Mcqs With Answers Pdf
Chemistry Mid Familia Form One
Chemistry Mock Papers
Chemistry Module Form 5
Chemistry Multiple Choice Questions and Answers Cxc
Chemistry Multiple Choice Questions and Answers Pdf
Chemistry Multiple Choice Questions With Answers Pdf
Chemistry Note
Chemistry Note Form Two All Chapters
Chemistry Notes
Chemistry Notes and Guestion and Answear
Chemistry Notes and Syllabus
Chemistry Notes Class 10
Chemistry Notes for Class 11 Pdf
Chemistry Notes for Class 12 Pdf
Chemistry Notes for High School Students
Chemistry Notes for IGCSE 2014
Chemistry Notes Form 1
Chemistry Notes Form 1 4
Chemistry Notes Form 1 Free Download
Chemistry Notes Form 1 KLB
Chemistry Notes Form 1 Pdf
Chemistry Notes Form 1-4
Chemistry Notes Form 1-4(1) Chemistry
Chemistry Notes Form 14
Chemistry Notes Form 2
Chemistry Notes Form 2 KLB
Chemistry Notes Form 2 Pdf
Chemistry Notes Form 2; Chemistry Notes
Chemistry Notes Form 3
Chemistry Notes Form 3 KLB
Chemistry Notes Form 3 Pdf
Chemistry Notes Form 4
Chemistry Notes Form 4 Chapter 2
Chemistry Notes Form 4 KLB
Chemistry Notes Form 4 Pdf
Chemistry Notes Form 4-pdf
Chemistry Notes Form Four
Chemistry Notes Form Four KLB
Chemistry Notes Form Four Pdf
Chemistry Notes Form One
Chemistry Notes Form One KLB
Chemistry Notes Form One Pdf
Chemistry Notes Form One to Form Four
Chemistry Notes Form Three
Chemistry Notes Form Three KLB
Chemistry Notes Form Three Pdf
Chemistry Notes Form Two
Chemistry Notes Form Two KLB
Chemistry Notes Form Two Pdf
Chemistry Notes Form2
Chemistry Notes IGCSE
Chemistry Notes Kenya
Chemistry Notes on Agroforestry
Chemistry Notes Pdf
Chemistry Notes:
Chemistry Objective Answer
Chemistry Objective Answer 2018
Chemistry Objective Questions for Competitive Exams
Chemistry Objective Questions for Competitive Exams Pdf
Chemistry Oral Exam Questions
Chemistry Paper 1
Chemistry Paper 1 2018 Marking Rules
Chemistry Paper 1 Notes
Chemistry Paper 1 Questions
Chemistry Paper 1 Questions and Answers
Chemistry Paper 1 Topics
Chemistry Paper 1 With Answers
Chemistry Paper 2
Chemistry Paper 2 2017
Chemistry Paper 2 2018 Marking Rules
Chemistry Paper 2 Questions and Answers
Chemistry Paper 2 Questions and Answers Pdf
Chemistry Paper 2 Revision
Chemistry Paper 2 Topics
Chemistry Paper 2018
Chemistry Paper 3 2018 Marking Rules
Chemistry Paper 3 Question and Answer
Chemistry Paper 3 Question Paper 2014 KCSE
Chemistry Paper 3 Question Paper 2015 KCSE
Chemistry Paper 3 Question Paper 2016 KCSE
Chemistry Paper 3 Question Paper 2017 KCSE
Chemistry Paper 3 Question Paper 2018 KCSE
Chemistry Paper 3 Questions and Answers
Chemistry Paper One Questions and Answers
Chemistry Paper One Topics
Chemistry Paper Two Qestions With Answers
Chemistry Paper1
Chemistry Paper2
Chemistry Paper3
Chemistry Paper4
Chemistry Past Papers
Chemistry Past Papers 2017
Chemistry Past Papers a Level
Chemistry Past Papers Form 1
Chemistry Past Papers Form 2
Chemistry Past Papers Form 3
Chemistry Past Papers O Level
Chemistry Pdf Download
Chemistry Pp1 KCSE 2016
Chemistry Practical Book Class 12 Pdf
Chemistry Practical Exam
Chemistry Practicals Form One
Chemistry Practicals Questions and Answers
Chemistry Practice Test 9th Grade
Chemistry Practice Test Answers
Chemistry Practice Test Questions and Answers
Chemistry Practice Test Quizlet
Chemistry Predicted Questions This Year KCSE
Chemistry Preparation Notes
Chemistry Pretest High School Pdf
Chemistry Question and Answer With Explanation
Chemistry Question and Answers 2019
Chemistry Question and Answers 2020
Chemistry Question and Answers 2021
Chemistry Question and Answers 2022
Chemistry Question and Answers Note
Chemistry Questions
Chemistry Questions and Answers
Chemistry Questions and Answers for High School
Chemistry Questions and Answers for High Schools
Chemistry Questions and Answers for High Schools Pdf
Chemistry Questions and Answers for Secondary Schools
Chemistry Questions and Answers Form 1
Chemistry Questions and Answers Form 2
Chemistry Questions and Answers Form 3
Chemistry Questions and Answers Form 4
Chemistry Questions and Answers Multiple Choice
Chemistry Questions and Answers Notes
Chemistry Questions and Answers O
Chemistry Questions and Answers Online
Chemistry Questions and Answers Pdf
Chemistry Questions and Answers Pdf for Class 12
Chemistry Questions and Answers Pdf for Competitive Exams
Chemistry Questions and Answers-form 2
Chemistry Questions for High School
Chemistry Questions for High School Students With Answers
Chemistry Questions for Senior 1
Chemistry Questions for Senior 2
Chemistry Questions for Senior 3
Chemistry Questions for Senior 4
Chemistry Questions for Senior 5
Chemistry Questions for Senior 6
Chemistry Questions for Senior Five
Chemistry Questions for Senior Four
Chemistry Questions for Senior One
Chemistry Questions for Senior Six
Chemistry Questions for Senior Three
Chemistry Questions for Senior Two
Chemistry Questions Form One
Chemistry Questions Multiple Choice
Chemistry Questions Quizlet
Chemistry Questions to Ask Your Teacher
Chemistry Quetion and Answer Form Four
Chemistry Quetion and Answer Form One
Chemistry Quetion and Answer Form Three
Chemistry Quetion and Answer Form Two
Chemistry Quiz for Class 9
Chemistry Quiz for Class 9 Chemistry
Chemistry Quiz Questions and Answers for Class 10
Chemistry Quiz Questions and Answers for Class 10 Pdf
Chemistry Quiz Questions and Answers for Class 12
Chemistry Quiz Questions and Answers for Class 9
Chemistry Quiz Questions and Answers for Class 9 Pdf
Chemistry Quiz Questions and Answers for High School
Chemistry Quiz Questions and Answers Multiple Choice
Chemistry Quiz Questions and Answers Pdf
Chemistry Quiz Questions for Class 12
Chemistry Quiz Questions for College Students
Chemistry Quiz With Answers
Chemistry Quiz With Answers Pdf
Chemistry Quizlet
Chemistry Revision
Chemistry Revision a Level
Chemistry Revision Chemistry Notes Chemistry
Chemistry Revision Exam
Chemistry Revision Examination
Chemistry Revision Form One
Chemistry Revision Notes
Chemistry Revision Notes Chemistry
Chemistry Revision Notes Form 1
Chemistry Revision Notes Form 2
Chemistry Revision Notes Form 3
Chemistry Revision Notes Form 4
Chemistry Revision Notes IGCSE
Chemistry Revision Paper One
Chemistry Revision Questions
Chemistry Revision Questions and Answers
Chemistry Revision Questions and Answers Form 1
Chemistry Revision Questions and Answers Form 2
Chemistry Revision Questions and Answers Form 3
Chemistry Revision Questions and Answers Form 4
Chemistry Revision Questions and Answers Form Four
Chemistry Revision Questions and Answers Form One
Chemistry Revision Questions and Answers Form Three
Chemistry Revision Questions and Answers Form Two
Chemistry Revision Questions Form 1
Chemistry Revision Questions Form 2
Chemistry Revision Questions Form 3
Chemistry Revision Questions Form 4
Chemistry Revision Questions Form Four
Chemistry Revision Questions Form One
Chemistry Revision Questions Form Three
Chemistry Revision Questions Form Two
Chemistry Revision Quiz
Chemistry Revision Test
Chemistry Secondary School Revision
Chemistry Simple Notes
Chemistry Spm Notes Download
Chemistry Spm Notes Pdf
Chemistry Spm Questions
Chemistry Study Form 2
Chemistry Study Guide
Chemistry Study Guide Answer Key
Chemistry Study Guide Answers
Chemistry Study Guide Chemistry Questions and Answers
Chemistry Study Guide Ib
Chemistry Study Guide Pdf
Chemistry Study Guides
Chemistry Study Notes
Chemistry Study Notes Materials Form 1 Pdf
Chemistry Study Notes Materials Form 2 3 Pdf
Chemistry Study Notes Materials Form 2 Pdf
Chemistry Study Notes Materials Form 3 Pdf
Chemistry Study Notes Materials Form 4 Pdf
Chemistry Syllabus in Kenya
Chemistry Syllabus Pdf
Chemistry Test 1 Quizlet
Chemistry Test Questions
Chemistry Test Questions and Answers
Chemistry Test Questions and Answers Pdf
Chemistry Topic One Form Four
Chemistry Topics Form One
Chemistry Unit 1 Quiz
Chemistry Vol 3
Chemistry | Revision Chemistry
Chemistry,form 4
Chemistry.form Four.topic Three
ChemistryExam Form Three
ChemistryModule Form 5
ChemistryNotes
ChemistryNotes for Class 11 Pdf
ChemistryNotes for Class 12 Pdf
ChemistryNotes Form 1
ChemistryNotes Form 1 Free Download
ChemistryNotes Form 2
ChemistryNotes Form 3
ChemistryNotes Form 3 Pdf
ChemistryNotes IGCSE
ChemistryNotes Pdf
ChemistryPast Papers
ChemistryQuestions and Answers Pdf
ChemistrySimple Notes
ChemistrySpm Notes Download
ChemistrySpm Notes Pdf
ChemistrySpm Questions
ChemistryStudy Guide Answers
ChemistryStudy Guide Pdf
ChemistryStudy Guides
Blologytextpapers
Bridge Chemistry
Business Past KCSE Past Papers
Business Studies Form 3 Notes Pdf
Business Studies Form 4 Notes Pdf
C R E Form One KLB
C R E Form One Oli Topic
C.r.e Form 1 Notes Kenya
C.r.e Form 2 Notes Kenya
C.r.e Form 3 Notes
C.r.e Form 3 Notes Kenya
C.r.e Form 3 Pdf
C.r.e Form 4 Notes Kenya
C.r.e Form One Notes Pdf
C.r.e Notes Form 1
C.r.e Revision Notes
C.r.e Short Notes
Cambridge IGCSE Chemistry
Cambridge IGCSE Chemistry 3rd Edition
Cambridge IGCSE Chemistry 3rd Edition Plus Cd South Asia Edition
Cambridge IGCSE Chemistry Answers
Cambridge IGCSE Chemistry Coursebook Pdf Download
Cambridge IGCSE Chemistry Practical Workbook
Cambridge IGCSE Chemistry Revision Guide Pdf
Cambridge IGCSE Chemistry Study and Revision Guide 2nd Edition Pdf
Cambridge IGCSE Chemistry Study and Revision Guide Pdf
Cambridge IGCSE Chemistry Workbook Free Download
Cambridge IGCSE Chemistry Workbook Pdf
Cambridge IGCSE® Chemistry Coursebook
Caucasian Chalk Circle Essay Questions
Chapter 1 Introduction to Chemistry
Chapter 1 Introduction to Chemistry Studies
Cie a Level Chemistry Notes 2016
Cie a Level Chemistry Notes Pdf
Cie Past Papers
Class 10 Chemistry Chapter 1 Mcqs
Class 8 Chemistry Notes KCSE-kcse
College Chemistry Notes
College Chemistry Practice Test
College Chemistry Quiz
College Chemistry Quiz Chapter 1
College Chemistry Quizlet
College Chemistry Study Guide
College Chemistry Study Guide Pdf
College Chemistry Test Questions and Answers
College Chemistry Volume 3 Pdf
College ChemistryNotes
Complete Chemistry for Cambridge IGCSE
Complete Chemistry for Cambridge IGCSE Revision Guide Pdf
County Mocks 2017
Cse Past Papers Chemistry 2017
Dl Chemistry Form 3 Pdf Kusoma
Download Chemistry Form 1
Download Chemistry Form 2
Download Chemistry Form 2 Notes
Download Chemistry Form 3
Download Chemistry Form 3 Notes
Download Chemistry Form 4
Download Chemistry Form Four
Download Chemistry Form One
Download Chemistry Form Three
Download Chemistry Form Two
Download Chemistry Notes Form 3
Download Chemistry Notes Form One
Download ChemistryNotes Form 3
Download Form Three Chemistry Notes
Download Free KCSE Past Papers Chemistry
Download Free KCSE Past Papers From KNEC.
Download KCSE Past Papers With Answers
Download KCSE Revision Notes
Download KLB Chemistry Book 2
Download KLB Chemistry Book 3
Download KLB Chemistry Book 4
Download Notes of Chemistry
Downloads | Chemistry | Form Four Exams | Exams
Downloads | Chemistry | Form One Exams | Exams
Downloads | Chemistry | Form Three Exams | Exams
Downloads | Chemistry | Form Two Exams | Exams
Downloads | KCSE Papers and Marking Schemes |
Dvance KCSE Past Papers
Easy Chemistry Questions
Edexcel a Level Chemistry B
Edexcel a Level Chemistry Notes Pdf
Edexcel a Level Chemistry Salters Nuffield
Edexcel A2 Chemistry Notes
Edexcel as Chemistry Revision Guide Pdf
Edexcel Chemistry A2 Revision Notes Pdf
Edexcel Chemistry Unit 2 Revision Notes
Edexcel GCSE Chemistry Revision Guide Pdf
Edexcel IGCSE Chemistry Past Papers
Edexcel IGCSE Chemistry Revision Guide Free Pdf Download
Edexcel IGCSE Chemistry Revision Guide Pdf
Edexcel IGCSE Chemistry Revision Guide Pdf Download
Electronics Form Four Notes
Energy Questions Chemistry Bowl
Essay Questions and Answers KCSE Chemistry Notes
Essay Questions and Answers on Betrayal in the City
Essay Questions Based on Betrayal in the City
Evolving World Chemistry Book 1 Pdf
Evolving World Chemistry Book 4 Notes
Evolving World Chemistry Book Form 1
Evolving World-history Book 3
Exam Notes for Chemistry 101
Exams KCSE Chemistry Paper 1 Questions and Answers
F3 Chemistry Test Paper
Find Download KCSE Past Papers With Answers
Find KCSE Chemistry Essay Questions and Answers
Form 1 Chemistry Exam
Form 1 Chemistry Notes
Form 1 Chemistry Questions and Answers
Form 1 Chemistry Questions and Answers Pdf
Form 1 Chemistry Revision Notes
Form 1 Chemistry Summurized Revision Pdf
Form 1 Chemistry Syllabus
Form 1 Chemistry Test Paper Pdf
Form 1 Chemistry Topics
Form 1 ChemistryNotes
Form 1 ChemistryQuestions and Answers
Form 1 ChemistryRevision Notes
Form 1 ChemistrySyllabus
Form 1 ChemistryTest Paper Pdf
Form 1 Past Papers
Form 1 Past Papers With Answers
Form 1 Revision Papers
Form 1 Subjects in Kenya
Form 2 Chemistry Exam
Form 2 Chemistry Exam Paper
Form 2 Chemistry Exam Paper 2016
Form 2 Chemistry Exam Paper Free Download
Form 2 Chemistry Exam Paper With Answer
Form 2 Chemistry Final Year Exam Paper 2
Form 2 Chemistry Notes
Form 2 Chemistry Notes and Revision Questions
Form 2 Chemistry Notes Pdf
Form 2 Chemistry Past Papers
Form 2 Chemistry Questions
Form 2 Chemistry Questions and Answers
Form 2 Chemistry Questions and Answers >
Form 2 Chemistry Questions and Answers Pdf
Form 2 Chemistry Revision Notes
Form 2 Chemistry Short Notes
Form 2 Chemistry Syllabus
Form 2 ChemistryExam Paper
Form 2 ChemistryExam Paper Free Download
Form 2 ChemistryExam Paper With Answer
Form 2 ChemistryFinal Year Exam Paper 2
Form 2 ChemistryPast Papers
Form 2 ChemistryRevision Notes
Form 2 ChemistryShort Notes
Form 2 ChemistrySyllabus
Form 2 Revision Papers
Form 2 Subjects in Kenya
Form 3 Chemistry Book
Form 3 Chemistry Exam
Form 3 Chemistry Exam Paper
Form 3 Chemistry Notes
Form 3 Chemistry Past Papers
Form 3 Chemistry Questions
Form 3 Chemistry Questions and Answers
Form 3 Chemistry Questions and Answers Pdf
Form 3 Chemistry Revision Notes
Form 3 Chemistry Syllabus
Form 3 ChemistryExam Paper
Form 3 ChemistryNotes
Form 3 ChemistryPast Papers
Form 3 ChemistryQuestions
Form 3 ChemistryQuestions and Answers Pdf
Form 3 ChemistryRevision Notes
Form 3 ChemistrySyllabus
Form 3 C.r.e
Form 3 Notes of Chemistry Topic on Fish
Form 3 Past Papers
Form 3 Revision Papers
Form 3 Subjects in Kenya
Form 4 Chemistry Exam
Form 4 Chemistry Notes
Form 4 Chemistry Notes Pdf
Form 4 Chemistry Questions and Answers
Form 4 Chemistry Questions and Answers Pdf
Form 4 Chemistry Revision Notes
Form 4 Chemistry Syllabus
Form 4 Chemistry Topics
Form 4 ChemistryNotes
Form 4 ChemistryRevision Notes
Form 4 ChemistrySyllabus
Form 4 ChemistryTopics
Form 4 Exam Papers
Form 4 Revision Papers
Form 4 Subjects in Kenya
Form 5 Chemistry Topics
Form 5 ChemistryTopics
Form Five Chemistry Notes
Form Five ChemistryNotes
Form Four Chemistry Book
Form Four Chemistry Notes
Form Four Chemistry Notes Pdf
Form Four Chemistry Questions and Answers
Form Four Chemistry Questions and Answers Pdf
Form Four Chemistry Revision Questions
Form Four Chemistry Syllabus
Form Four Chemistry Topics
Form Four ChemistryNotes
Form Four ChemistryQuestions and Answers
Form Four ChemistryQuestions and Answers Pdf
Form Four ChemistryTopics
Form Four Notes
Form Four Revision Papers
Form Four Subjects in Kenya
Form One Chemistry Book
Form One Chemistry Examination
Form One Chemistry First Topic
Form One Chemistry Lesson Plan
Form One Chemistry Notes Pdf
Form One Chemistry Past Papers Pdf
Form One Chemistry Questions
Form One Chemistry Questions and Answers
Form One Chemistry Questions and Answers Pdf
Form One Chemistry Revision Questions
Form One Chemistry Short Notes
Form One Chemistry Syllabus
Form One Chemistry Topics
Form One ChemistryExamination
Form One ChemistryPast Papers Pdf
Form One ChemistryQuestions and Answers
Form One ChemistryQuestions and Answers Pdf
Form One ChemistryTopics
Form One Exams
Form One Notes of Chemistry
Form One Past Papers
Form One Subjects in Kenya
Form One Term One Chemistry Exam
Form One Term One ChemistryExam
Form Three Chemistry Book
Form Three Chemistry Book Pdf
Form Three Chemistry Notes
Form Three Chemistry Notes Pdf
Form Three Chemistry Questions and Answers
Form Three Chemistry Questions and Answers Pdf
Form Three Chemistry Revision Questions
Form Three Chemistry Syllabus
Form Three Chemistry Topics
Form Three ChemistryNotes
Form Three ChemistryNotes Pdf
Form Three ChemistryQuestions and Answers
Form Three ChemistryQuestions and Answers Pdf
Form Three ChemistryTopics
Form Three Subjects in Kenya
Form Two Chemistry Book
Form Two Chemistry Cat
Form Two Chemistry Examination
Form Two Chemistry Notes
Form Two Chemistry Notes Pdf
Form Two Chemistry Past Papers
Form Two Chemistry Questions and Answers
Form Two Chemistry Questions and Answers Pdf
Form Two Chemistry Revision Questions
Form Two Chemistry Syllabus
Form Two Chemistry Topics
Form Two ChemistryNotes
Form Two ChemistryNotes Pdf
Form Two ChemistryQuestions and Answers
Form Two ChemistryQuestions and Answers Pdf
Form Two ChemistrySyllabus
Form Two ChemistryTopics
Form Two Notes
Form Two Subjects in Kenya
Free a-level Chemistry Revision App | Pass Your Chemistry Exams
Free Chemistry Form 1 Notes
Free Chemistry Notes Form 1
Free Chemistry Notes Pdf
Free ChemistryNotes Pdf
Free College Chemistry Practice Test
Free Form1,form2,form3 Past Papers Free KCSE Past Papers
Free KCSE Mocks 2015
Free KCSE Past Papers 2014
Free KCSE Past Papers KCSE Past
Free KCSE Past Papers Kenya,
Free KCSE Past Papers With Answers
Free KCSE Questions and Answers on Chemistry
Free KCSE Revision Notes
Free Marking Schemes
Free Mocks Online KCSE Answers Past Exams Question Papers
Free Revision Papers
From Three Notes Topic One KLB
Fun Chemistry Questions
Funny Chemistry Questions
Funny Chemistry Questions and Answers
Funny Chemistry Questions to Ask
Funny Chemistry Quotes
GCSE Chemistry Exam Questions and Answers
GCSE Chemistry Past Papers
GCSE Chemistry Revision
GCSE Chemistry Revision Notes
GCSE Chemistry Revision Notes Pdf
GCSE Chemistry Revision Notes Pdf 9-1
GCSE Chemistry Revision Questions and Answers
GCSE Chemistry Textbook Pdf
GCSE Chemistry Topics Pass My Exams: Easy Exam Revision Notes
General Chemistry Notes Pdf
General Chemistry Practice Test With Answers
General Chemistry Quiz
General Chemistry Quiz Pdf
General Chemistry Test Questions and Answers
General Chemistry Test Questions and Answers Pdf
General Knowledge in Chemistry Human Body
Good Chemistry Questions to Ask
GRE Chemistry Practice Test
GRE Chemistry Subject Test Pdf
Handbook of Chemistry Pdf Free Download
Hard Chemistry Questions
Hard Chemistry Questions and Answers
Hard Chemistry Questions to Ask Your Teacher
Hard Chemistry Quiz Questions
Hard Form 3 Chemistry Question
High School Chemistry Final Exam Doc
High School Chemistry Final Exam Pdf
High School Chemistry Final Exam Questions
High School Chemistry Final Exam Questions and Answers
High School Chemistry Notes
High School Chemistry Practice Test
High School Chemistry Pretest With Answers
High School Chemistry Questions and Answers Pdf
High School Chemistry Study Guide
High School Chemistry Test Questions and Answers Pdf
High School ChemistryNotes
High School ChemistryStudy Guide
How to Answer KCSE Chemistry Question
How to Motivate a Form 4 Student
How to Motivate a KCSE Candidate
How to Motivate a KCSE Student
How to Pass Chemistry Questions & Answers Form 1&2 | Text Book
How to Revise Chemistry
How to Revise Effectively for KCSE
How to Study Chemistry: 5 Study Techniques to Master Chemistry
Hsc Chemistry 2018
Hsc Chemistry 2019
Https://www.knec.ac.ke/ Www.knec-portal.ac.ke/ KNEC Portal:
Ial Chemistry Notes
Ib Chemistry Cold War Notes
Ib Chemistry Notes
Ib Chemistry Notes Pdf
Ib Chemistry of the Americas Notes
Ib Chemistry of the Americas Study Guide
Ib Chemistry Paper 2 Study Guide
Ib Chemistry Question Bank by Topic
Ib Chemistry Study Guide Pdf
Ict Notes Form 1
IGCSE Chemistry Alternative to Practical Revision
IGCSE Chemistry Alternative to Practical Revision Notes
IGCSE Chemistry Book
IGCSE Chemistry Book Pdf Download
IGCSE Chemistry Notes
IGCSE Chemistry Notes 2017 Pdf
IGCSE Chemistry Notes Edexcel
IGCSE Chemistry Paper 2 Notes
IGCSE Chemistry Paper 6 Notes
IGCSE Chemistry Past Papers
IGCSE Chemistry Past Papers 2014
IGCSE Chemistry Past Papers 2017
IGCSE Chemistry Pdf
IGCSE Chemistry Pre Release Material 2018
IGCSE Chemistry Resources
IGCSE Chemistry Revision Guide
IGCSE Chemistry Revision Guide Free Download
IGCSE Chemistry Revision Guide Pdf Download
IGCSE Chemistry Revision Notes Pdf
IGCSE Chemistry Revision Worksheets
IGCSE Chemistry Workbook Pdf
IGCSE Chemistry Znotes
IGCSE ChemistryPast Papers
IGCSE Notes Chemistry
Importance of Agroforestry
Inorganic Chemistry Multiple Choice Questions With Answers Pdf
Inorganic Chemistry Questions and Answers Pdf
Interesting Chemistry Questions
Interesting Chemistry Questions and Answers
Interesting Questions to Ask About Chemistry
Intro to Chemistry Quiz
Introduction of Chemistry Form One
Introduction to Chemistry
Introduction to Chemistry Notes
Introduction to Chemistry Pdf
Introduction to ChemistryNotes
Is Agroforestry Sustainable?
K.c.s.e Answers Chemistry Paper One 2018
K.c.s.e Chemistry 2017
K.c.s.e Chemistry 2018
K.c.s.e Chemistry Paper 1 2017
K.c.s.e Mocks 2018
K.c.s.e Papers 2015
K.c.s.e Papers 2016
K.c.s.e Past Papers 2014
K.c.s.e.Chemistry Paper 2 Year 2018
K.c.s.e.results 2018 for Busia County
K.l.b Chemistry Form 3
K.l.b Chemistry Notes
K.l.b ChemistryNotes
Kasneb Past Papers for Colleges Chemistry Past Papers
KCSE 2010 Marking Scheme
KCSE 2010 Past Papers
KCSE 2011 Chemistry Paper 1
KCSE 2011 Marking Scheme
KCSE 2012 Chemistry Paper 2 Marking Scheme
KCSE 2012 Marking Schemes
KCSE 2013 Chemistry Paper 1
KCSE 2013 Marking Scheme
KCSE 2013 Marking Scheme Pdf
KCSE 2014
KCSE 2015 Chemistry Paper 2
KCSE 2015 Chemistry Paper 3
KCSE 2015 Marking Scheme
KCSE 2015 Past Papers
KCSE 2016 Chemistry Paper 1
KCSE 2016 Chemistry Paper 2
KCSE 2017 Chemistry Paper 1
KCSE 2017 Chemistry Paper 2
KCSE 2017 Hostory Papers With Answers.com
KCSE 2017 Marking Scheme
KCSE 2017 Papers
KCSE 2017 Papers and Marking Scheme
KCSE 2017 Papers Pdf
KCSE 2017 Past Papers
KCSE 2017 Prediction Pdf
KCSE 2018 Chemistry and Answers
KCSE 2018 Chemistry Prediction
KCSE 2018 Leakage
KCSE 2018 Marking Scheme
KCSE 2018 Papers
KCSE 2018 Prediction Pdf
KCSE 2018 Predictions
KCSE 2018 Questions
KCSE 2018 Questions and Answers
KCSE 2019 Leakage Chemistry
KCSE 2019 Marking Scheme
KCSE 2019 Questions
KCSE 2019 Questions and Answers
KCSE 2020 Questions
KCSE 2020 Questions and Answers
KCSE Answers
KCSE Answers Past Exams Question Papers Downloads |
KCSE Chemistry 2011
KCSE Chemistry 2016
KCSE Chemistry Diagramsbiology Revision Tips
KCSE Chemistry Essay Questions and Answers
KCSE Chemistry Essay Questions and Answers Pdf
KCSE Chemistry Essays
KCSE Chemistry Essays Pdf
KCSE Chemistry Marking Schemes
KCSE Chemistry Notes
KCSE Chemistry Notes Pdf
KCSE Chemistry Notes, Syllabus, Questions, Answers
KCSE Chemistry Paper 1
KCSE Chemistry Paper 1 2011
KCSE Chemistry Paper 1 2012
KCSE Chemistry Paper 1 2013
KCSE Chemistry Paper 1 2015
KCSE Chemistry Paper 1 2016
KCSE Chemistry Paper 1 2017
KCSE Chemistry Paper 1 2017 Pdf
KCSE Chemistry Paper 1 Questions and Answers
KCSE Chemistry Paper 2
KCSE Chemistry Paper 2 2012
KCSE Chemistry Paper 2 2012 KCSE Chemistry Paper 2 2015
KCSE Chemistry Paper 2 2013
KCSE Chemistry Paper 2 2014
KCSE Chemistry Paper 2 2015
KCSE Chemistry Paper 2 2016
KCSE Chemistry Paper 2 2017
KCSE Chemistry Paper 3
KCSE Chemistry Paper 3 2012
KCSE Chemistry Paper 3 2016
KCSE Chemistry Paper 3 2017
KCSE Chemistry Paper 3 Past Papers
KCSE Chemistry Past Papers
KCSE Chemistry Past Papers and Answers
KCSE Chemistry Past Papers Pdf
KCSE Chemistry Practical
KCSE Chemistry Practical 2015
KCSE Chemistry Practical 2016
KCSE Chemistry Practical Past Papers
KCSE Chemistry Practicals
KCSE Chemistry Practicals KCSE Chemistry Paper 1
KCSE Chemistry Question and Answer
KCSE Chemistry Questions and Answers
KCSE Chemistry Questions and Answers Ap Chemistry
KCSE Chemistry Revision
KCSE Chemistry Revision Notes
KCSE Chemistry Revision Papers
KCSE Chemistry Revision Questions
KCSE Chemistry Revision Questions and Answers
KCSE Chemistry Syllabus
KCSE ChemistryNotes
KCSE ChemistryPaper 1
KCSE ChemistryPaper 2
KCSE ChemistryPaper 2 Pdf
KCSE ChemistrySyllabus
KCSE Business Paper 1 2016
KCSE Business Past Papers
KCSE Business Studies Past Papers
KCSE Essay Questions in Betrayal in the City
KCSE Essays
KCSE Exam Papers 2018
KCSE Exam Papers Answers
KCSE Form 1 Chemistry Revision
KCSE Form 2 Chemistry Revision
KCSE Form 3 Chemistry Revision
KCSE Form 4 Chemistry Revision
KCSE Form Four Chemistry Revision
KCSE Form One Chemistry Revision
KCSE Form Three Chemistry Revision
KCSE Form Two Chemistry Revision
KCSE KCSE Past Papers KNEC
KCSE Leakage
KCSE Leakage Chemistry
KCSE Made Familiar Chemistry
KCSE Made Familiar Chemistry Pdf
KCSE Marking Scheme 2016
KCSE Marking Schemes
KCSE Marking Schemes 2017
KCSE Marking Schemes Pdf
KCSE Mock Exams
KCSE Mock Papers 2015
KCSE Mock Papers 2017
KCSE Mock Papers 2018
KCSE Mock Papers Pdf
KCSE Mock Papers Pdf 2018
KCSE Mock Papers Pdf KCSE Past Papers
KCSE Mocks 2017
KCSE Mocks 2018
KCSE Notes
KCSE Online Notes
KCSE Online Past Papers
KCSE Online Registration
KCSE Papers 2015
KCSE Papers and Marking Schemes | Exams
KCSE Past Papers
KCSE Past Papers 2007
KCSE Past Papers 2009
KCSE Past Papers 2010
KCSE Past Papers 2011
KCSE Past Papers 2011 Pdf
KCSE Past Papers 2012
KCSE Past Papers 2013
KCSE Past Papers 2013knec
KCSE Past Papers 2014
KCSE Past Papers 2014 Pdf
KCSE Past Papers 2015
KCSE Past Papers 2015 Marking Schemes
KCSE Past Papers 2015 Pdf
KCSE Past Papers 2016
KCSE Past Papers 2016 Pdf
KCSE Past Papers 2017
KCSE Past Papers 2017 Pdf
KCSE Past Papers 2018
KCSE Past Papers Chemistry
KCSE Past Papers Chemistry and Answers
KCSE Past Papers Chemistry Pdf
KCSE Past Papers Chemistry With Answers
KCSE Past Papers Chemistryand Answers
KCSE Past Papers Business Studies and Answers
KCSE Past Papers KCSE and Answers
KCSE Past Papers KCSE and Answers Free Mocks Online
KCSE Past Papers Marking Scheme
KCSE Past Papers Pdf Download
KCSE Past Papers Pdf Download KCSE 2013
KCSE Past Papers With Answers
KCSE Past Papers Woodwork and Answers
KCSE Prediction 2017
KCSE Prediction 2018
KCSE Prediction 2018 Pdf
KCSE Prediction Papers 2018
KCSE Prediction Questions
KCSE Prediction Questions 2018
KCSE Prediction Questions and Answers
KCSE Questions
KCSE Questions and Answers
KCSE Questions and Answers.
KCSE Questions on Chemistry
KCSE Results, Online Registration, KCSE Result Slip.
KCSE Revision
KCSE Revision Notes
KCSE Revision Notes Chemistry
KCSE Revision Notes Pdf
KCSE Revision Papers
KCSE Revision Papers 2014
KCSE Revision Papers With Answers
KCSE Revision Question for Chemistry
KCSE Revision Questions
KCSE Revision Questions and Answers
KCSE Revision | Secondary School | Text Books | Text Book Centre
KCSE Syllabus Pdf
KCSE Trial 2017
KCSE Trial Exams 2017
Kenya Secondary School Chemistry Syllabus
Kenya Secondary School Chemistry Syllabus Pdf
Kenya Secondary School ChemistrySyllabus Pdf
Kenya Secondary School Syllabus Pdf
Kenya-kcse-christian Religious Education Syllabus
Kenyaplex KCSE Past Papers
Kenyaplex Past Papers for Secondary
KLB Chemistry Book 1 Download
KLB Chemistry Book 1 Notes
KLB Chemistry Book 1 Pdf
KLB Chemistry Book 2
KLB Chemistry Book 2 Notes
KLB Chemistry Book 2 Notes Pdf
KLB Chemistry Book 2 Pdf
KLB Chemistry Book 3 Notes
KLB Chemistry Book 3 Pdf
KLB Chemistry Book 3 Pdf Download
KLB Chemistry Book 4 Notes
KLB Chemistry Book 4 Pdf
KLB Chemistry Book 4 Pdf Download
KLB Chemistry Book 4 Topics
KLB Chemistry Book One
KLB Chemistry Form 1
KLB Chemistry Form 1 Notes
KLB Chemistry Form 1 Pdf
KLB Chemistry Form 2
KLB Chemistry Form 2 Book
KLB Chemistry Form 2 Notes
KLB Chemistry Form 2 Pdf
KLB Chemistry Form 2 Pdf Download
KLB Chemistry Form 2 Schemes of Work
KLB Chemistry Form 3
KLB Chemistry Form 3 Notes
KLB Chemistry Form 3 Notes Pdf
KLB Chemistry Form 3 Pdf
KLB Chemistry Form 3 Pdf Download
KLB Chemistry Form 4
KLB Chemistry Form 4 Notes
KLB Chemistry Form 4 Pdf
KLB Chemistry Form Four
KLB Chemistry Form Four Notes
KLB Chemistry Form One
KLB Chemistry Form One Notes
KLB Chemistry Form Three
KLB Chemistry Form Three Notes
KLB Chemistry Form Two
KLB Chemistry Form Two Notes
KLB Chemistry Notes
KLB Chemistry Notes Form 4
KLB Chemistry Pdf
KLB ChemistryNotes
KLB ChemistryNotes Form 4
KLB ChemistryPdf
KNEC Chemistry Syllabus
KNEC Examiners Portal KNEC Website
KNEC Ict Past Papers
KNEC Past Papers for Colleges
KNEC Past Papers Free Download
KNEC Past Papers Free Downloads
KNEC Past Papers Pdf
KNEC Portal Confirmation
KNEC Portal KCSE Results
KNEC Portal KNEC Past Papers for Colleges Kasneb Past Papers
KNEC Revision Papers
KNEC Technical Exams Past Papers
Kusoma Chemistry Notes
Kusoma Chemistry Notes Pdf
Kusoma Notes Chemistry
Kusoma.co.ke
Kusoma.com Past Papers
Learner Guide for Cambridge IGCSE Chemistry
Longhorn Chemistry Book 3 Pdf
Made Familiar Chemistry
Made Familiar Chemistry Pdf
Made Familiar Chemistry Questions
Maktaba Tetea Notes
Marking Scheme KCSE Chemistry Past Papers
Math Form2 Note
Mcqs About Gaseous Exchange
Middle School Chemistry Bowl Chemistry Questions
Mock Past Papers 2017
Mock Past Papers With Answers
Mokasa Mock 2017
More Than 1800 Chemistry Questions and Answers to Help You Study
Multiple Choice Questions on Chemistry
Necta Chemistry Past Papers
Necta Chemistry Practicals
Necta ChemistryPast Papers
Necta ChemistryPracticals
Necta Form Four Past Papers
Necta Past Papers Form 4
Necta Past Papers Form 4 2016
Necta Past Papers Form Six
Necta Past Papers Form Two
Necta Questions and Answers
Necta Review Questions
Notes Chemistry Form 1
Notes Chemistry Form 2
Notes Chemistry Form 3
Notes Chemistry Form 3 Notes Pdf
Notes Chemistry Form 3 Syllabus
Notes Chemistry Form 4 Syllabus
Notes on Chemistry Studies
Notes Za Chemistry 4m 2
Notes Za Chemistry Form One
Notes Za Chemistry Form Three
O Level Chemistry Practical Experiments
O Level Chemistry Questions and Answers Pdf
Orm Three Chemistry Notes
Page Navigation
Papacambridge Chemistry IGCSE
Papers KNEC KCSE Online Past
Papers KNEC KCSE Results Past Papers
Past KCSE Papers
Past Paper Questions by Topic Chemistry
Past Papers 2014
Past Papers in Kenya
Pdf Chemistry Form 3
Pdf Chemistry Notes
Pdf Chemistry Notes Form 1
Pdf Chemistry Notes Form 2
Pdf Chemistry Notes Form 3
Pdf Chemistry Notes Form 4
Pdf Chemistry Notes Form Four
Pdf Chemistry Notes Form One
Pdf Chemistry Notes Form Three
Pdf Chemistry Notes Form Two
Pdf Form 1 Chemistry Questions and Answers
Pdf Form 2 Chemistry Questions and Answers
Pdf Form 3 Chemistry Questions and Answers
Pdf Form 4 Chemistry Questions and Answers
Pdf Form Four Chemistry Questions and Answers
Pdf Form One Chemistry Questions and Answers
Pdf Form Three Chemistry Questions and Answers
Pdf Form Two Chemistry Questions and Answers
Pdf Free KCSE Past Papers and Marking Schemes
Pdf" Revision Questions Chemistry Form 1
Practical Chemistry Experiments Pdf
Practical Chemistry Question and Answer Pdf
Pre Mocks 2018
Preliminary Chemistry
Primary and Secondary Tillage Implements Ppt
Pte KNEC Past Papers
Questions and Answers Pdf Chemistry Form 1
Questions and Answers Pdf Chemistry Form 2
Questions and Answers Pdf Chemistry Form 3
Questions and Answers Pdf Chemistry Form 4
Questions and Answers Pdf Chemistry Form Four
Questions and Answers Pdf Chemistry Form One
Questions and Answers Pdf Chemistry Form Three
Questions and Answers Pdf Chemistry Form Two
Questions Based to Introduction to Chemistry
Questions on Gaseous Exchange in Humans
Questions on Introduction to Chemistry
Questions to Ask in Chemistry Class
Questions to Confuse Your Chemistry Teacher
Quizlet Chemistry Test
Quizlet Test Questions
Qustions in Chemistry and Answers
Revision
Revision Chemistry Notes and Questions?
Revision Quiz for Chemistry for Form Three
S.1 Chemistry Questions
S.2 Chemistry Questions
S.3 Chemistry Questions
S.4 Chemistry Questions
Sample Essays on Betrayal in the City
School Chemistry Notes
Secondary Chemistry Notes
Secondary Chemistry Notes Pdf
Secondary ChemistryNotes Pdf
Senior 1 Chemistry Notes
Senior 2 Chemistry Notes
Senior 3 Chemistry Notes
Senior 4 Chemistry Notes
Senior 5 Chemistry Notes
Senior 6 Chemistry Notes
Senior Five Chemistry Notes
Senior Four Chemistry Notes
Senior One Chemistry Notes
Senior Six Chemistry Notes
Senior Three Chemistry Notes
Senior Two Chemistry Notes
Simple Scientific Questions
Smart Questions to Ask a Chemistry Teacher
Snab Chemistry Revision Notes
Southwest Mock Paper 2 2016 Chemistry Only
Spm Chemistry Revision Notes
Spm Notes
Success Chemistry Spm Pdf
Success ChemistrySpm Pdf
Summary of Chemistry Form 3
Tahossa Past Papers
To Motivate a Form 4 KCSE Student
To Motivate a Form 4 Student
Topical Revision Material
Tricky Chemistry Questions and Answers
Tricky Chemistry Questions for Adults
Tricky Chemistry Questions With Answers
Tricky Chemistry Quiz Questions
Two Chemistry Revision Questions
University Chemistry Volume 3 Openstax
University Chemistry Volume 3 Pdf
University Chemistry Volume 4 Pdf
Ur Revision Guide IGCSE Chemistry
What Are the Types of Gametes
Working of Excretory System
Www.Chemistry Form One Notes.com
Www.Chemistry From One KLB.com
Www.form 1 Chemistry.com
Www.form 2 Chemistry.com
Www.form 3 Chemistry.com
Www.form 4 Chemistry.com
Www.form Four Chemistry.com
Www.form One Chemistry.com
Www.form Three Chemistry.com
Www.form Two Chemistry.com
Www.kusoma Notes
Www.kusoma Revision Materials
Www.kusoma.co.ke Chemistry Notes
Xtremepapers IGCSE Chemistry
Year 11 Chemistry
Z Notes Chemistry IGCSE
Znotes as Chemistry
Acids and Bases Chem Revision Questions
Chemistry Notes Combine Form 1, 2, 3 and 4
Chemistry Notes Form 3
A Level Chemistry Questions and Answers Pdf
All Chemistry Questions and Answers Pdf,ppt
Chemistry Exam Questions and Answers Pdf
Chemistry Multiple Choice Questions and Answers Pdf
Chemistry Questions and Answers for High Schools Pdf
Chemistry Notes Form 1-4 Pdf
Free High School Notes Kenya
Free Kcse Revision Notes
General Chemistry Test Questions and Answers Pdf
High School Chemistry Multiple Choice Questions and Answers Pdf
Kcse Chemistry Revision Notes Pdf
Kcse Revision Books Pdf
Kcse Revision Notes Pdf
Kenya Secondary School Notes Pdf
Notes of Form 123 and 4 All Subject
Chemistry Notes Form 1 Free Download
Advice to KCSE Candidates
Best Revision Books for KCSE
How to Pass an Exam Successfully
How to Pass KCSE 2018
How to Pass KCSE 2019
How to Pass KCSE Chemistry Paper
How to Pass KCSE Chemistry
How to Pass KCSE Chemistry
How to Pass KCSE 2019
K.c.s.e Chemistry Paper 1 2017
KCSE 2019 Prediction
KCSE Prediction 2019
KCSE Revision Tips
KCSE Chemistry Paper 1 2018
KCSE Chemistry Paper 2 2018
KCSE Chemistry Past Papers Pdf
KCSE Past Papers 2012
KCSE Past Papers 2017 Pdf
KCSE Past Papers 2018
KCSE Past Papers of Chemistry Pp2
KCSE Chemistry Past Papers Pdf
Chemistry KCSE Papers With Their Marking Schemes
Chemistry Paper 1 and Answers
KCSE 2017 Papers and Marking Scheme
KCSE 2019 Papers and Marking Scheme
KCSE Chemistry Paper 1 2018
KCSE Chemistry Paper 1 2019
KCSE Chemistry Paper 2 2017
KCSE Chemistry Paper 2 2018
KCSE Chemistry Paper 2 2019
KCSE Chemistry Past Papers and Answers
KCSE Marking Schemes Pdf
KCSE Past Papers 2015 Marking Schemes
KCSE Past Papers 2019 Marking Schemes
Chemistry KCSE Papers With Their Marking Schemes
Chemistry Paper 1 and Answers
KCSE 2017 Papers and Marking Scheme
KCSE 2019 Papers and Marking Scheme
KCSE Chemistry Paper 1 2018
KCSE Chemistry Paper 1 2019
KCSE Chemistry Paper 1 2019 Past Papers
KCSE Chemistry Paper 2 2017
KCSE Chemistry Paper 2 2018
KCSE Chemistry Paper 2 2019
KCSE Chemistry Paper 2 2019 Past Papers
KCSE Chemistry Paper 3 2019 Past Papers
KCSE Chemistry Past Papers and Answers
KCSE Marking Schemes Pdf
KCSE Past Papers 2015 Marking Schemes
KCSE Past Papers 2019 Marking Schemes
KCSE Past Papers Chemistry Paper 1 2019
KCSE Past Papers Chemistry Paper 2 2019
KCSE Past Papers Chemistry Paper 3 2019
Past Papers KCSE Chemistry Paper 1 2019
Past Papers KCSE Chemistry Paper 2 2019
Past Papers KCSE Chemistry Paper 3 2019
Chemistry Form 1 Questions and Answers
Chemistry Paper 1 2018
Chemistry Paper 2 2018
Chemistry Paper 1 2019
Chemistry Paper 2 2019
Chemistry Paper 1 Notes
Chemistry Paper 1 Questions and Answers Pdf
Chemistry Paper 2 Questions and Answers
Chemistry Questions and Answers Form 2
KCSE Chemistry Paper 1 2018
KCSE Chemistry Paper 2 2018
KCSE Chemistry Paper 1 2017
KCSE Chemistry Paper 2 2017
KCSE Chemistry Paper 1 2019
KCSE Chemistry Paper 2 2019
Most Tested KCSE Chemistry Questions
4m1 Notes Viusasa
4m2 Notes Viusasa
4m3 Notes Viusasa
4m4 Notes Viusasa
Download on Viusasa - Download Now for Free
Elimu - Viusasa
Elimu Library | Notes, Exams, Lesson Plans, Schemes
Elimu Online
High School Notes - Revision Materials for Kenyan Schools
Https //www.viusasa.com/elimu Kenya
Notes Viusasa Elimu Form 1
Notes Viusasa Elimu Form 2
Notes Viusasa Elimu Form 3
Notes Viusasa Elimu Form 4
Notes Viusasa Elimu Form Four
Notes Viusasa Elimu Form One
Notes Viusasa Elimu Form Three
Notes Viusasa Elimu Form Two
Viusasa
Viusasa Education
Viusasa Elimu
Viusasa Elimu Class 6
Viusasa Elimu Form 1
Viusasa Elimu Form 1 Notes
Viusasa Elimu Form 2
Viusasa Elimu Form 2 Notes
Viusasa Elimu Form 3
Viusasa Elimu Form 3 Notes
Viusasa Elimu Form 4
Viusasa Elimu Form 4 Notes
Viusasa Elimu Form Four
Viusasa Elimu Form Four Notes
Viusasa Elimu Form One
Viusasa Elimu Form One Notes
Viusasa Elimu Form Three
Viusasa Elimu Form Three Notes
Viusasa Elimu Form Two
Viusasa Elimu Form Two Notes
Viusasa High School Notes - Revision Materials for Kenyan Schools
Viusasa Notes
Chemistry Mock Papers
Chemistry Paper 1 2019
Chemistry Paper 1 Notes
Chemistry Paper 1 Questions and Answers
Chemistry Paper 1 Questions and Answers Pdf
Chemistry Paper 2 Form 3
Chemistry Paper 2 Notes
Chemistry Paper 2 Questions and Answers Pdf
Chemistry Past Papers
Chemistry Past Papers Pdf
Chemistry Questions and Answers Pdf
Chemistry Revision Questions and Answers Pdf
Chemistry Revision Questions Pdf
Common Exam Questions in Chemistry Paper 1
Common Exam Questions in Chemistry Paper 2
Common Test Questions in Chemistry Paper 1
Common Tested Questions in Chemistry Paper 1
Commonly Tested Questions in Chemistry Paper 1
K.c.s.e.Chemistry Questions and Answers
KCSE 2019 Chemistry Paper 1 Marking Scheme
KCSE 2019 Chemistry Paper 2
KCSE 2019 Chemistry Paper 2 Marking Scheme
KCSE 2020 Prediction Questions and Answers
KCSE Chemistry Paper 1 2019
KCSE Chemistry Paper 2 2016
KCSE Chemistry Paper 2 2017
KCSE Chemistry Paper 2 2018
KCSE Chemistry Paper 2 2019
KCSE Chemistry Questions and Answers
Most Tested Questions in Chemistry Paper 1
Most Tested Questions in Chemistry Paper 2
Mostly Tested Questions in Chemistry Paper 1
Mostly Tested Questions in Chemistry Paper 2
Chemistry Form 4
Chemistry Revision Questions Form 1
Combined Science Notes Form 1
Form 4 Notes
High Flyer Series Chemistry Form 1-4
Kcse Past Papers Chemistry With Answers
Chemistry Notes Download
Secondary Chemistry Notes Pdf
Water and Hydrogen Form 1 Notes
High Flyer Series KCSE Revision in Chemistry
High Flyer Series KCSE Revision Chemistry Form 1-4 Revised
A Level Chemistry Notes Uganda Pdf Download
Buddo Junior School Holiday Work
Chemistry Notes for a Level Pdf
Chemistry Notes Form 1
Chemistry Notes Form 3 the Mole
Chemistry Notes Form 4
Chemistry Notes O Level
Chemistry Notes O Level Uganda
Chemistry Notes O Level Uganda Pdf
Chemistry Notes Pdf
Download Chemistry Notes
Form 3 Chemistry Notes
Form 3 Chemistry Questions and Answers
Form Three Chemistry Syllabus Pdf
Gayaza High School Chemistry Notes
Gayaza High School Chemistry Notes Pdf
Gayaza High School Chemistry Past Papers
Gayaza High School Chemistry Past Papers Answers
Gayaza High School Chemistry Past Papers Questions and Answers
Gayaza High School E Learning
Gayaza High School Elearning Platform
Gayaza High School Examinations
Gayaza High School Holiday Work
Gayaza High School Notes
Gayaza High School Notes 2021
Gayaza High School Notes 2022
Gayaza High School Notes 2023
Gayaza High School Notes Pdf
Gayaza Junior School E Learning Platform
Gayaza Junior School Holiday Work
Gayaza O Level Chemistry Notes
Home › Gayaza High School | Elearning Platform
Kabojja Junior School Holiday Work Pdf
Klb Chemistry Book 3
Klb Chemistry Form 3 Teachers Guide
Klb Chemistry Notes
List of Ugandan E-learning Platforms for Students
Mirembe Junior School Holiday Work
Ntare School Past Papers
O Level Chemistry Notes Free Download
O Level Chemistry Notes in Uganda
O Level Chemistry Notes Pdf
O Level Chemistry Notes Uganda Pdf
O Level Chemistry Notes Uganda Pdf Download
O Level General - Home › Gayaza High School | Elearning
S.1 Chemistry Notes | Standard High School Zzana
S.3 Chemistry Notes
S.4 Chemistry 2 Notes | Standard High School Zzana
Seeta High School Elearning
Seeta High School Holiday Work
Seeta High School Notes
Seeta High School Notes Pdf
Seeta High School Past Papers
Seeta High School Past Papers Pdf
Senior 1 Chemistry Notes
Senior 1 Chemistry Notes in Uganda
Senior 1 Chemistry Notes Uganda
Senior 1 Chemistry Questions
Senior 1 Exams
Senior 1 Work
Senior 1 Work 2020
Senior 1 Work 2020 Uganda
Senior 2 Chemistry Notes
Senior 2 Chemistry Notes in Uganda
Senior 2 Chemistry Notes Uganda
Senior 2 Chemistry Questions
Senior 2 Exams
Senior 2 Work
Senior 2 Work 2020
Senior 2 Work 2020 Uganda
Senior 3 Chemistry Notes
Senior 3 Chemistry Notes in Uganda
Senior 3 Chemistry Notes Uganda
Senior 3 Chemistry Questions
Senior 3 Exams
Senior 3 Work
Senior 3 Work 2020
Senior 3 Work 2020 Uganda
Senior 4 Chemistry Notes
Senior 4 Chemistry Notes in Uganda
Senior 4 Chemistry Notes Uganda
Senior 4 Chemistry Questions
Senior 4 Exams
Senior 4 Work
Senior 4 Work 2020
Senior 4 Work 2020 Uganda
Senior Four Chemistry Notes
Senior Four Chemistry Notes in Uganda
Senior Four Chemistry Notes Uganda
Senior Four Chemistry Questions
Senior Four Exams
Senior Four Work
Senior Four Work 2020
Senior Four Work 2020 Uganda
Senior One Chemistry Notes
Senior One Chemistry Notes in Uganda
Senior One Chemistry Notes Uganda
Senior One Chemistry Questions
Senior One Exams
Senior One Work
Senior One Work 2020
Senior One Work 2020 Uganda
Senior Three Chemistry Notes
Senior Three Chemistry Notes in Uganda
Senior Three Chemistry Notes Uganda
Senior Three Chemistry Questions
Senior Three Exams
Senior Three Work
Senior Three Work 2020
Senior Three Work 2020 Uganda
Senior Two Chemistry Notes
Senior Two Chemistry Notes in Uganda
Senior Two Chemistry Notes Pdf
Senior Two Chemistry Notes Uganda
Senior Two Chemistry Questions
Senior Two Exams
Senior Two Work
Senior Two Work 2020
Senior Two Work 2020 Uganda
St Mary's Kitende E Learning
Standard High School Notes
Standard High School Zana a Level Notes
Standard High School Zana Com Notes
Standard High School Zana E Learning
Standard High School Zana E Learning Platform
Standard High School Zana E-learning Platform
Standard High School Zana Notes for Senior Two
Standard High School Zana Notes Pdf
Standard High School Zana Website
Standard High Zana Notes
Uace Chemistry Notes
Uace Chemistry Notes Pdf
Uce Chemistry Notes Pdf
Uce Chemistry Notes Pdf Download
Uce Past Papers
Uganda Secondary Schools E-learning Platform
Uneb Marking Guides Pdf
Uneb Past Papers and Answers Pdf
Air and Combustion Form 1 Notes
Chemistry Form 1 Notes Pdf Free Download
Chemistry Form One Questions and Answers
Chemistry Notes Form 1 Easy Elimu
Chemistry Notes Form 1-4 Pdf
Chemistry Notes Form 1-4 Pdf Download Free
High School Chemistry Notes Pdf
Klb Chemistry Form 1 Notes Pdf Download
Notes of Form One Chemistry Laboratory and Drawings
Chemistry Form 1 Questions and Answers Pdf Download
Chemistry Notes Form 1-4
Chemistry Form 1 Questions and Answers Pdf
Form 1 Chemistry Topical Questions
Form 1 Chemistry Exam Paper With Answer Pdf
Form 1 Exams 2020
Form 1 Chemistry Past Papers
Form 1 Chemistry Revision Papers With Answers
Sample Chemistry Test for Form One Exams
Ntare School Past Papers
Seeta High School Notes Pdf
Seeta High School Past Papers
Seeta High School Past Papers Pdf
St Mary's Kitende Past Papers
Uce Chemistry Notes Pdf
Uce Past Papers
Uneb Marking Guides Pdf
Uneb Past Papers and Answers Pdf
Chemistry Book 3 Download
Chemistry Form 3 Questions and Answers
Chemistry Form 3 Syllabus
Chemistry Form 3 Topics
Chemistry Notes Form 3
Chemistry Notes Form 3
Chemistry Notes Form 3
Chemistry Notes Form Three
Chemistry Notes Pdf Download
Form 3 Chemistry Exam
Form 3 Chemistry Notes on Pdf
Revision Chemistry Revision a Level Chemistry
Revision Notes - Chemistry
S.1-Chemistry-notes
S.1-Chemistry-notes Uganda
S.2-Chemistry-notes
S.2-Chemistry-notes Uganda
S.3-Chemistry-notes
S.3-Chemistry-notes Uganda
S.4-Chemistry-notes
S.4-Chemistry-notes Uganda
S.5-Chemistry-notes
S.5-Chemistry-notes Uganda
S.6-Chemistry-notes
S.6-Chemistry-notes Uganda
S1 Chemistry Notes Term 1
S1 Chemistry Notes Term 2
S1 Chemistry Notes Term 3
S2 Chemistry Notes Term 1
S2 Chemistry Notes Term 2
S2 Chemistry Notes Term 3
S3 Chemistry Notes Term 1
S3 Chemistry Notes Term 2
S3 Chemistry Notes Term 3
S4 Chemistry Notes Term 1
S4 Chemistry Notes Term 2
S4 Chemistry Notes Term 3
Senior 3 Chemistry Notes
Senior 3 Chemistry Notes Uganda
Senior Three Chemistry Notes
Senior Three Chemistry Notes Uganda
Chemistry Form 1 the Cell
Chemistry Form One Notes Free – Education News
Chemistry Notes Form 1-4
Chemistry Questions and Answers Form 1 - Chemistry Form One
Chemistry Questions and Answers Form 2 - Chemistry Form Two
Chemistry Questions and Answers Form 3 - Chemistry Form Three
Chemistry Questions and Answers Form 4 - Chemistry Form Four
Chemistry Questions and Answers Senior 1 - Chemistry Senior One
Chemistry Questions and Answers Senior 2 - Chemistry Senior Two
Chemistry Questions and Answers Senior 3 - Chemistry Senior Three
Chemistry Questions and Answers Senior 4 - Chemistry Senior Four
Chemistry Topic by Topic Questions and Answers
Combined Science Notes Form 1
Form 1 Chemistry Revision Questions and Answers
Form 1 Chemistry Topical Questions
Form 1 Revision Papers With Answers
Form 2 Chemistry Revision Questions and Answers
Form 3 Chemistry Revision Questions and Answers
Form Four Chemistry Revision Questions and Answers
Form One Chemistry Revision Questions and Answers
Form Three Chemistry Revision Questions and Answers
Form Two Chemistry Revision Questions and Answers
Free Chemistry Form 1 Notes
Free Chemistry Notes
Introduction to Chemistry Form One
Kcse Past Papers: Chemistry Form 1
Kcse Past Papers: Chemistry Form 1 Topical Questions
Kcse Past Papers: Chemistry Form 1 Topical Questions and Answers
Most Tested Areas in Chemistry Kcse
Most Tested Areas in Chemistry Kcse Exams
Most Tested Areas in Chemistry Uneb
Most Tested Areas in Form 1 in Chemistry
Most Tested Areas in Form 2 in Chemistry
Most Tested Areas in Form 3 in Chemistry
Most Tested Areas in Form 4 in Chemistry
Most Tested Areas in Form Four in Chemistry
Most Tested Areas in Form One in Chemistry
Most Tested Areas in Form Three in Chemistry
Most Tested Areas in Form Two in Chemistry
Most Tested Areas in Kcse Chemistry
Most Tested Areas in Kcse Chemistry Exams
Most Tested Areas in Senior 1 in Chemistry
Most Tested Areas in Senior 2 in Chemistry
Most Tested Areas in Senior 3 in Chemistry
Most Tested Areas in Senior 4 in Chemistry
Most Tested Areas in Senior Four in Chemistry
Most Tested Areas in Senior One in Chemistry
Most Tested Areas in Senior Three in Chemistry
Most Tested Areas in Senior Two in Chemistry
Most Tested Areas in Uneb Chemistry
Revision Notes Chemistry Form 1 - Free Kcse Past Papers
Who Was Chemistry Champion KCSE 2019
Top 100 Students in Chemistry KCSE 2019
Best 100 Students in Chemistry KCSE 2019
Top Student in Chemistry KCSE
Best Student in Chemistry KCSE
Who Was Chemistry Champion KCSE 2020
Top 100 Students in Chemistry KCSE 2020
Best 100 Students in Chemistry KCSE 2020
Names of Best Chemistry Students KCSE
Names of Top Chemistry Students KCSE
High School Chemistry Notes Pdf
Best High School Chemistry Notes Pdf
Comprehensive High School Chemistry Notes Pdf
Klb Chemistry Form 1 Book Pdf
Chemistry Form 1 Best Notes
Chemistry Form 1 Notes Pdf
Chemistry Form 1 Pressure
Chemistry Form 1 Questions and Answers
Chemistry Form 1 Questions and Answers Pdf Download
Chemistry Form 2 Best Notes
Chemistry Form 3 Best Notes
Chemistry Form 4 Best Notes
Chemistry Form Four Best Notes
Chemistry Form One Best Notes
Chemistry Form Three Best Notes
Chemistry Form Two Best Notes
Chemistry Notes Form 1 to 4
Chemistry Notes Pdf
Scholarship 2026/27
Current Scholarships 2026/2027 - Fully Funded
Full Undergraduate Scholarships 2026 - 2027
Fully Funded Masters Scholarships 2026 - 27
PhD Scholarships for International Students - Fully Funded!
Funding Opportunities for Journalists 2026/2027
Funding for Entrepreneurs 2026/2027
***
