KCSE Past Papers Chemistry 2011
Click Here - Free KCSE Past Papers » KCSE Past Papers Chemistry 2011 » Free Downloads » KCSE Papers & Marking Schemes
2.3.1 Chemistry Paper 1 (233/1)
1 (a) What name is given to the process by which alcohol is formed from a carbohydrate? (1 mark)
(b) Explain why the solubility of ethane in water is lower than that of ethanol. (2 marks)
2 Complete the nuclear equation below:
(a)
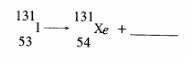

(c) Give one harmful effect of radioisotopes. (1 mark)
3 A mixture contains ammonium chloride, copper (II) oxide and sodium chloride.
Describe how each of the substances can be obtained from the mixture. (3 marks)
4 The set-up below shows how nitrogen gas is prepared in the laboratory.
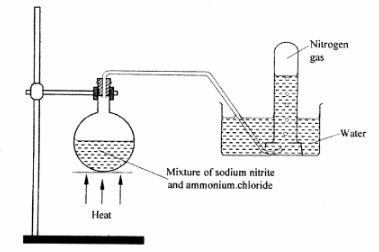
(a) Describe how nitrogen gas is formed in the flask. (2 marks)
(b) Nitrogen is inert. State one use of the gas based on this property. (1 mark)
5 The diagram below represents part of the periodic table. Use it to answer the questions that follow:
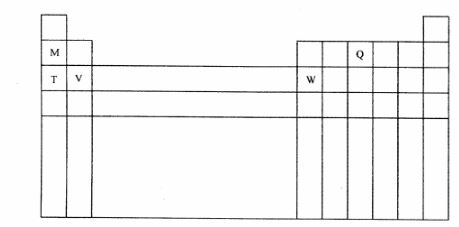
(a) Write the electronic arrangement for the stable ion formed by W. (1 mark)
(b) Write an equation for the reaction between V and Q. (1 mark)
(c) How do the ionisation energies of the elements M and T compare? Explain. (1 mark)
6 A certain mass of gas occupies 0.15dm3 at 293 K and 98,648.5Pa. Calculate its volume at 101325 Pa and 273 K. (2 marks)
7 When lead(ii) nitrate is heated. one of the products is a brown gas. (a) Write the equation of the reaction that occurs. (1 mark)
(b) If O.290dm3 of the brown gas was produced, calculate the mass of the lead(ii) nitrate that was heated. (R.F.M of lead (II) nitrate = 331; Molar gas volume = 24dm3). (2 marks)
8 (a) What is meant by a strong acid? (1 mark)
(b) In an experiment, 40cm3 of 0.5M hydrochloric acid was reacted with excess sodium carbonate and the volume of carbon (IV) oxide produced recorded with time.
In another experiment, the same volume and concentration of ethanoic acid was also reacted with excess sodium carbonate and the volume of carbon(iv) oxide produced recorded with time.
On the grid below, sketch and label the curves if the volumes of carbon (IV) oxide were plotted against time. (2 marks)
Volume of C02 cm3
9 State two reasons why hydrogen is not commonly used as a fuel. (2 marks)
10 During a class experiment, chlorine gas was bubbled into a solution of potassium iodide. (a) State the observations made. (1 mark)
(b) Using an ionic equation, explain why the reaction is redox. (2 marks)
11 Exhaust fumes of some cars contain carbon (II) oxide and other gases.
(a) Explain how carbon (II) oxide is formed in the internal combustion engines. (1 mark)
(b) Name two gases other than carbon (II) oxide that are contained in exhaust fumes and are pollutants. (2 marks)
12 Sodium hydroxide can be prepared by the following methods; I and II.
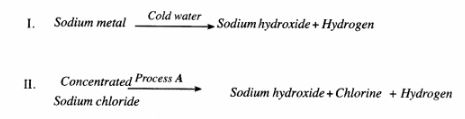
(a) Name one precaution that needs to be taken in method I. (1 mark)
(b) Give the name of process A. (1 mark)
(c) Give one use of sodium hydroxide. ( 1 mark)
13 Distinguish between the terms deliquescent and efflorescent as used in chemistry. (2 marks)
14 Two organic compounds P and Q clecolourise acidified potassium manganate (VII) solution; but only P reacts with sodium metal to give a colourless gas. Which homologous series does compound P belong? Give a reason. (2 marks)
15 Soap dissolves in water according to the equation below;
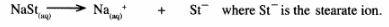
(a) Write the formula of the scum formed when soap is used in hard water. (1 mark)
(b) Write the ionic equation for the reaction that occurs when sodium carbonate is used to remove hardness in water. (1 mark)
16 Ethanoic acid and ethanol react as shown in the equation below:

Other than warming, how would the state of equilibrium be established within a short time? (1 mark)
17 The set up below was used to prepare a gas and study some of its properties. Study it and answer the questions that follow:
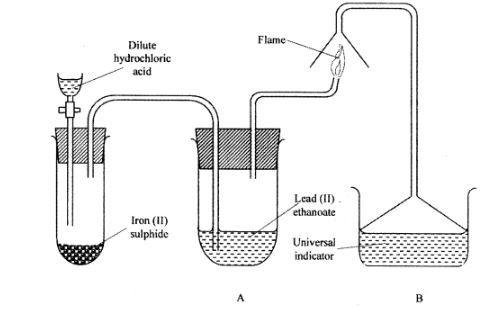
(a) State and explain the observations made in the:
(i) tube labelled A; (1 mark)
(ii) beaker labelled B. (1 mark)
(b) State one precaution that should be taken when carrying out this experiment. (1 mark)
18 Under certain conditions, chlorine gas reacts with sodium hydroxide to form sodium hypochloxite.
(a) Name the conditions under which sodium hydroxide reacts with chlorine to form sodium hypochlorite. (1 mark)
(b) State two uses of sodium hypochlorite. (2 marks)
19 50kg of ammonium sulphate (NH4)2 SO4 and 30kg of urea :CO(NH2)2 fertilizers were applied in two equal sizes of plots A and B to enrich their nitrogen content. Show by working, which plot was more enriched with nitrogen. (N = 14; S = 32; O = 16; C = 12; H = 1) (3 marks)
20 Describe how the PH of anti-acid (Actal) powder can be determined in the laboratory. (2 marks)
21 Graphite is one of the allotropes of carbon.
(a) Name one other element which exhibits allotropy. (1 mark)
22 The table below gives some properties of three elements in group (VII) of the periodic table.
Study it and answer the questions that follow:
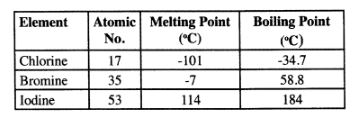
(a) Which element is in liquid fonn at room temperature? Give a reason. (1 mark)
(b) Explain why the boiling point of iodine is much higher than that of chlorine. (2 marks)
23 The thermalchemical reaction between carbon and sulphur is as shown by the equation below:
On the grid below, sketch and label the energy level diagram for the reaction. (2 marks)
24 The table below gives the number of electrons, protons and neutrons in substances X, Y and Z.
study it and answer the questions below
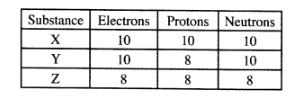
(a) Which letter represents an ion? (1 mark)
(b) Which of the substances are isotopes? Give a reason. (2 marks)
25 (a) State the Gay Lussac’s Law. (1 mark)
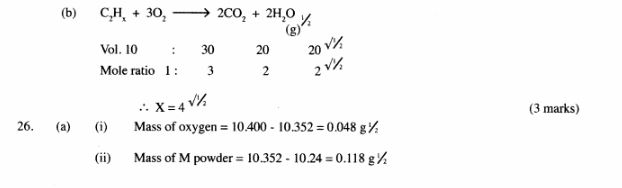
(b) 1Ocm3 of a gaseous hydrocarbon, C2HX required 30cm3 of oxygen for complete combustion. If steam and 20cm3 of carbon (iv) oxide were produced, what is the value of X? (2 marks)
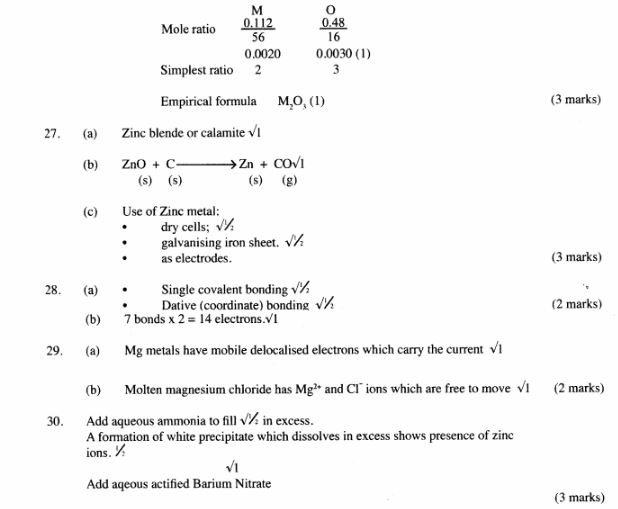
26 The data given below was recorded when metal M was completely burnt in air. M is not the actual symbol of the metal. (R.A.M; M = 56, O =16)
Mass of empty crucible and lid = 10.240g
Mass of crucible, lid and metal M = 10.352g
Mass of crucible, lid and metal oxide = l0.400g
(a) Determine the mass of: (2 mark)
(i) metal M;
(ii) oxygen. ( 2 mark)
(b) Determine the empirical formula of me metal oxide. (2 marks)
27 The flow chart below shows some processes involved in the industrial extraction of Zinc metal
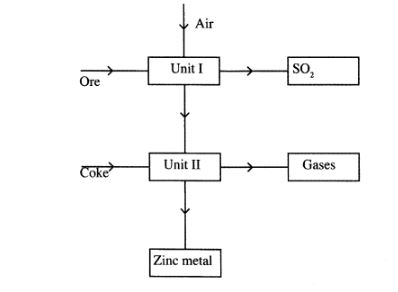
(a) Name one ore from which Zinc is extracted(1 marks)
(b) Write the equation of the reaction taking place in unit II(1 marks)
(c) Name two uses of zinc metal.(1 marks)
28 The diagram below shows the bonding between aluminium chloride and ammonia.
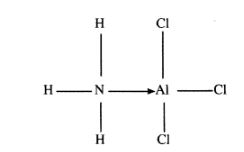
(a) Name the types of bonds that exist in the molecule.(1 marks)
(b) How many electrons are used for bonding in the molecule? (1 marks)
29. Explain why the following substances conduct an electric current.
(a) Magnesium metal.(1 marks)
(b) Molten magnesium chloride.(1 marks)
30 A sample of river water is suspectedc to contain zinc and sulphate ions.
Describe how the presence of zinc ions and sulphate ions can be established. (3 marks)
23.2 Chemistry Paper 2 (233/2)
1 The flow chart below shows some of the processes involved in large scale production of sulphuric (VI) acid. Use it to answer the questions that follow.
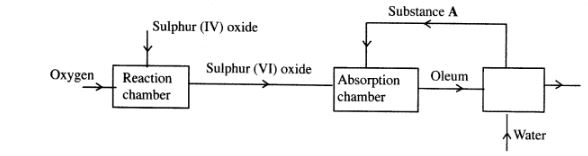
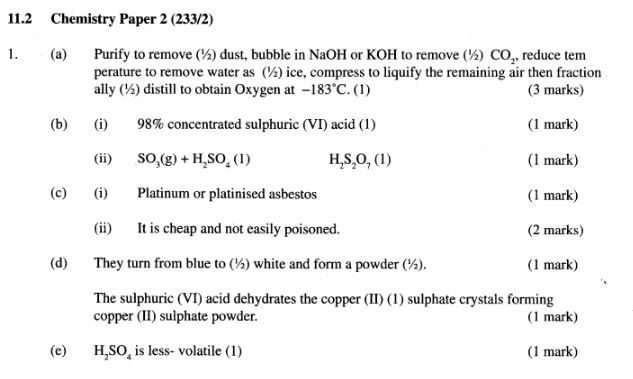
(a) Describe how oxygen is obtained from air on a large scale. (3 marks)
(b) (i) Name substance A. (1 mark)
(ii) Write an equation for the process that takes place in the absorption chamber. (1 mark)
(c) Vanadium (V) oxide is a commonly used catalyst in the contact process.
(i) Name another catalyst which can be used for this process. (1 mark)
(ii) Give two reasons why vanadium (V) oxide is the commonly used catalyst. (2 marks)
(d) State and explain the observations made when concentrated sulphuric (VI) acid is added to crystals of copper (II) sulphate in a beaker. (2 marks)
(e) The reaction of concentrated sulphuric (V1) acid with sodium chloride produces hydrogen chloride gas. State the property of concentrated sulphuric (Vi) acid illustrated in this reaction. (1 mark)
(f) Name four uses of sulphuric (VI) acid. (2 marks)
2 The set-up below was used by a student to investigate the products formed when aqueous copper (ll) chloride was electrolysed using carbon electrodes.
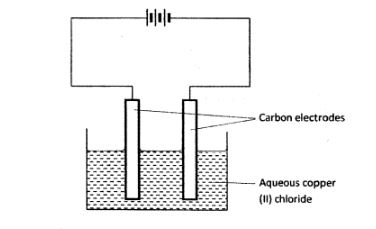
(a) (i) Write the equation for the reaction that takes place at the cathode. (1 mark) (ii) Name and describe a chemical test for the product initially formed at the anode when a highly concentrated solution of copper (ll) chloride is electrolysed. (3 marks)
(iii) How would the mass of the anode change if the carbon anode was replaced with copper metal? Explain. (2 marks)
(b) 0.6 g of metal B Were deposited when a current of O.45A was passed through an electrolyte for 72 minutes. Determine the charge on the ion of metal B. (Relative atomic mass of B = 59, 1 Faraday = 96 500 coulombs) (3 marks)
(c) The electrode potentials for cadmium and zinc are given below:
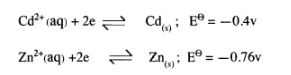
Explain why it is not advisable to store a solution of cadmium nitrate in a container made of zinc. (2 arks)
3 (a) Ethanol can be manufactured from ethene and steam as shown in the equation below:
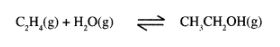
Temperature and pressure will affect the position of equilibrium of the above reaction. Name the other factor that will affect the position of equilibrium of the above reaction. (1 mark)
(b) The data in the table below was recorded when one mole of ethene was reacted with excess steam. The amount of ethanol in the equilibrium mixture was recorded under different conditions of temperature and pressure. Use the data to answer the questions that follow.
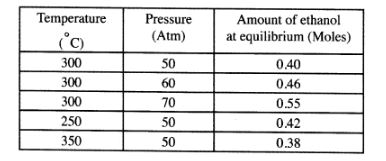
(i) State whether the reaction between ethene and steam is exothermic or endothermic. Explain your answer. (3 marks) .
(ii) State and explain one advantage and one disadvantage of using extremely high pressure in this reaction.
I. Advantage (2 marks)
II. Disadvantage (2 marks)
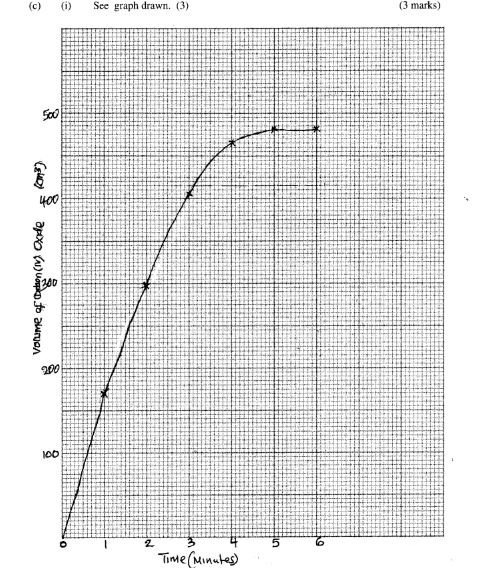
(c) In an experiment to determine the rate of reaction between calcium carbonate and dilute hydrochloric acid, 2g of calcium carbonate were reacted with excess 2 M hydrochloric acid. The volume of carbon (IV) oxide evolved was recorded at regular intervals of one minute for six minutes. The results are shown in the table below.

(i) Plot a graph of time in minutes on the horizontal axis against volume of carbon (IV) oxide on the vertical axis. (3 marks)
(ii) Determine the rate of reaction at 4 minutes. (2 marks)
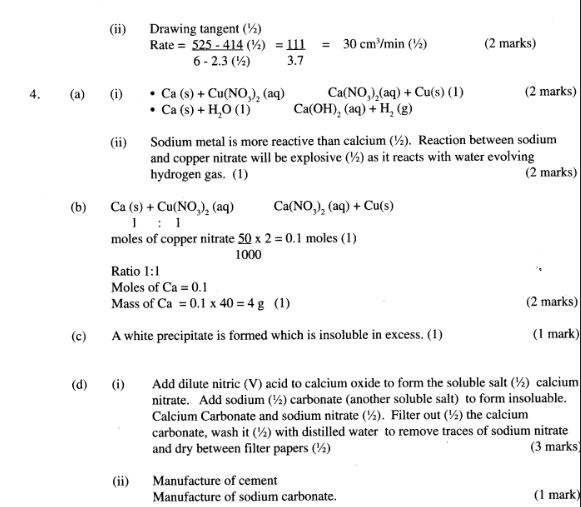
4 (a) When excess calcium metal was added to 50 cm3 of 2 M aqueous copper (II) nitrate in a beaker, a brown solid and bubbles of gas were observed.
(i) Write two equations for the reactions which occurred in the beaker. (2 marks)
(ii) Explain why it is not advisable to use sodium metal for this reaction. (2 marks)
(b) Calculate the mass of calcium metal which reacted with copper (II) nitrate solution. (Relative atomic mass of Ca = 40) (2 marks)
(c) The resulting mixture in (a) above was filtered and aqueous sodium hydroxide added to the filtrate dropwise until in excess. What observations were made?(1 mark)
(d) (i) Starting with calcium oxide, describe how a solid sample of calcium carbonate can be prepared. (3 marks)
(ii) Name one use of calcium carbonate. (1 mark)
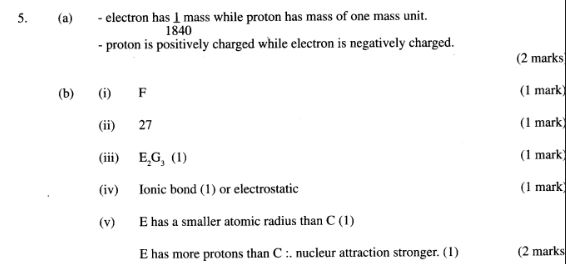
5. (a) Other than their location in the atom, name two other differences between an electron and a proton. (2 marks)
(b) The table below gives the number of electrons, protons and neutrons in particles A, B,C,D, E,F and G.
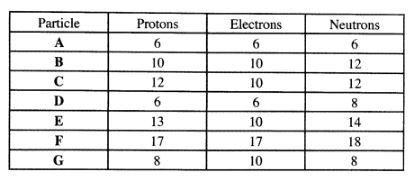
(i) Which particle is likely to be a halogen?(1 mark)
(ii) What is the mass number of E?(1 mark)
(iii) Write the formula of the compound formed when E combines with G.(1 mark)
(iv) Name the type of bond formed in (m) above.(1 mark)
(v) How does the radii of C and E compare? Give a reason.(2 marks)
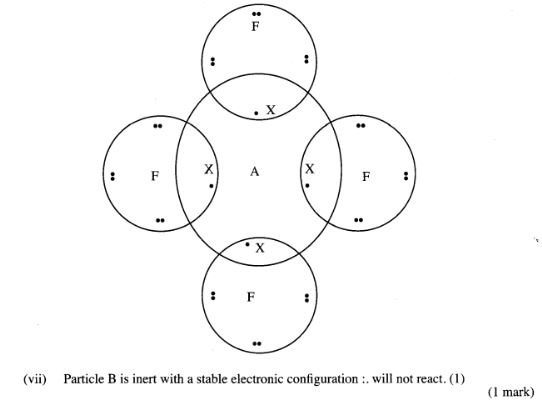
(vi) Draw a dot (.) and cross (X) diagram for the compound formed between A and F.(1 mark)
(vii) Why would particle B not react with particle D?(1 mark)
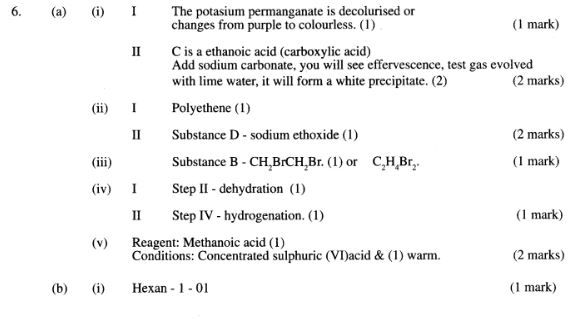
6 (a) Study the flow chart below and answer the questions that follow.
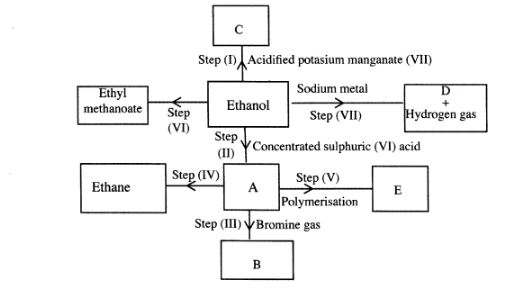
(i) I What observation will be made in Step I?(1 mark)
II Describe a chemical test that can be carried out to show the identity of compound C.(2 marks)
(ii) Give the names of the following:(2 marks)
I E ............................... II Substance D .................. (iii) Give the formula of substance B.(1 mark)
(iv) Name the type of reaction that occurs in:(1 mark)
I Step (II) ............................
II Step (IV) ............................
(v) Give the reagent and conditions necessary for Step (2 marks)(VI). Reagent: .......................
Conditions .................. ..
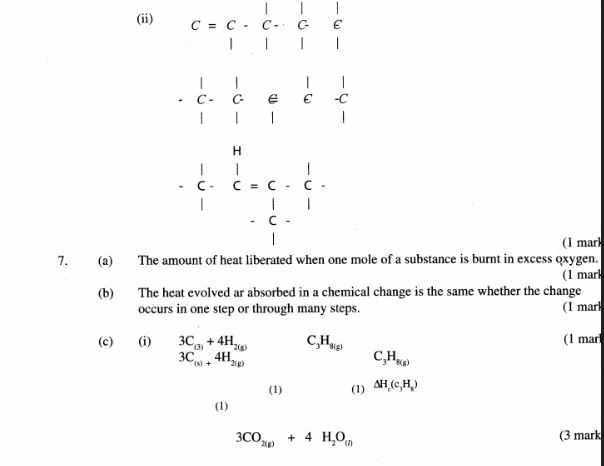
(b) (i) Name the following structure.
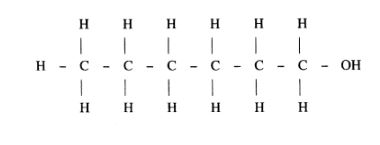
(ii) Draw the structure of an isomer of pentene. (1 mark)
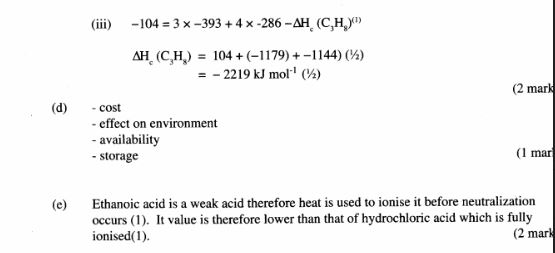
7 (a) What is meant by molar heat of combustion? (1 mark)
(b) State the Hess’s Law. (1 mark)
(c) Use the following standard enthalpies of combustion of graphite, hydrogen and enthalpy of formation of propane.
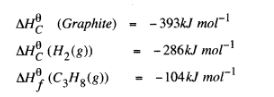
(i) Write the equation for the formation of propane. (1 mark)
(ii) Draw an energy cycle diagram that links the heat of formation of propane with its heat of combustion and the heats of combustion of graphite and hydrogen. (3 marks)
(iii) Calculate the standard heat of combustion of propane. (2 marks)
(d) Other than the enthalpy of combustion, state one factor which should be considered when choosing a fuel. (1 mark)
(e) The molar enthalpies of neutralization for dilute hydrochloric acid and dilute nitric (V)acid are -57.2k.j/mol while that of ethanoic acid is —55.2kJ/mol. Explain this observation. (2 marks)
3.3.3 Chemistry Paper 3 (233/3)
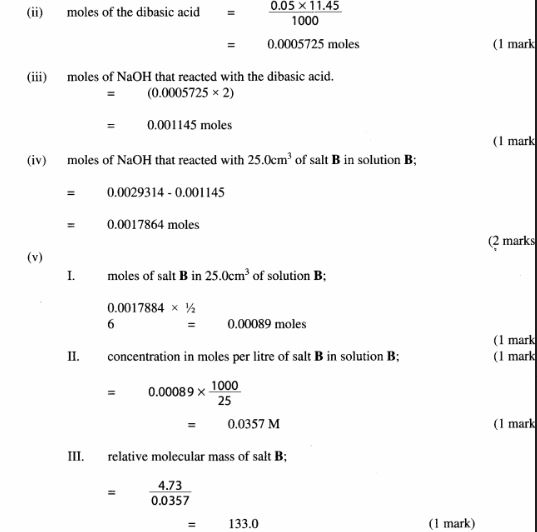
1 You are provided with:
- 1.6Og of solid A. a dibasic acid.
- Solution B containing 4.75g per litre of salt B.
- Aqueous sodium hydroxide. solution C.
- Phenolphthalein indicator.
You are required to prepare a solution of solid A and use it to determine the:-
- Concentration of sodium hydroxide. solution C
- React salt B with excess sodium hydroxide and then determine the relative molecular mass of salt B.
Procedure I
(a) Using a burette, place 25 .Ocm3 of solution B in each of two 250ml conical flasks.
Using a pipette and pipette filler, add 25.0cm3 of solution C to each of the two conical flasks. (The sodium hydroxide added is in excess). Label the conical flasks 1 and 2.
(b) Heat the contents of the first conical flask to boiling and then let the mixture boil for 5 minutes. Allow the mixture to cool.
(c) Repeat procedure (b) with the second conical flask.
While the mixtures are cooling, proceed with procedure II.
Procedure II
(a) Place all of solid A in 21 250 ml volumetric flask. Add about 150cm3 of distilled water, shake well to dissolve the solid and then add water to make up to the mark. Label this as solution A.
(b) Place solution A in a clean burette. Using a pipette and pipette filler, place 25 .0cm3 of solution C in a 250ml conical flask. Add 2 drops of phenolphthalein indicator and titrate with solution A. Record your results in Table 1. Repeat the titration two more times and complete the table.
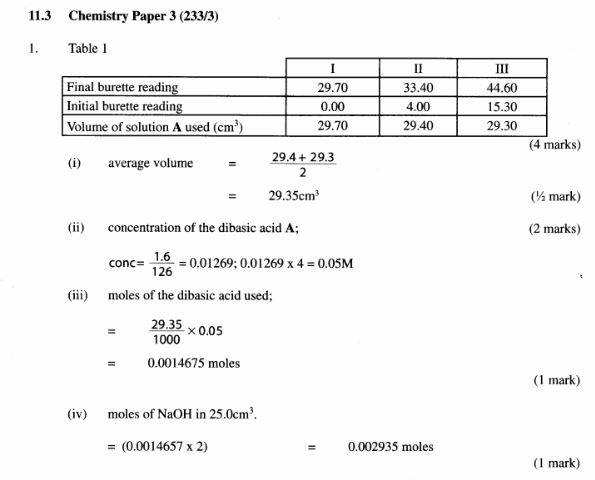
Table 1
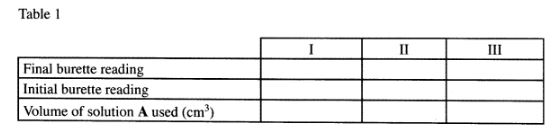
(4 marks)
Calculate the:-
(i) average volume of solution A used: (1/2 mark)
(ii) concentration in moles per litre of the dibasic acid in solution A; (2 marks) (Relative molecular mass of A is 126).
(iii) moles of the dibasic acid used; (1 mark)
(iv) moles of sodium hydroxide in 25 .0cm3 of solution C. (l mark)
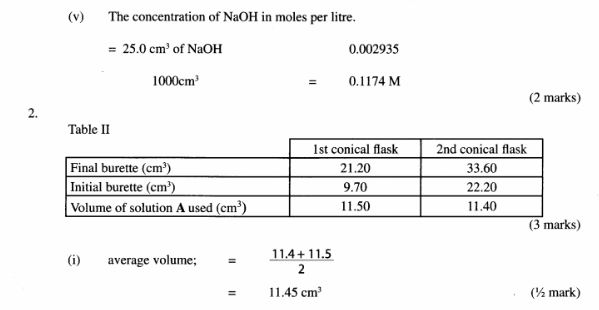
(v) concentration of sodium hydroxide in moles per litre. (2 marks)
Procedure III
Add 2 drops of phenolpthalein indicator to the contents of the first conical flask prepared in procedure l and titrate with solution A. Record your results in Table 2. Repeat the procedure with the contents of the second conical flask and complete the table.
Table 2
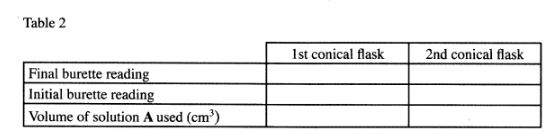
(3 marks)
Calculate the:-
(i) average volume of solution A used; (1/2 mark)
(ii) moles of the dibasic acid used; (1 mark)
(iii) moles of sodium hydroxide that reacted with the dibasic acid. (1 mark)
(iv) moles of sodium hydroxide that reacted with 25 .0cm3 of salt B in solution B; (2 marks)
(v) Given that l mole of salt B reacts with 2 moles of sodium hydroxide, calculate the: l. number of moles of salt B in 25.0cm3 of solution B; (1 mark)
ll. concentration in moles per litre of salt B in solution B; (1 mark)
lll. relative molecular mass of salt B; (2 marks)
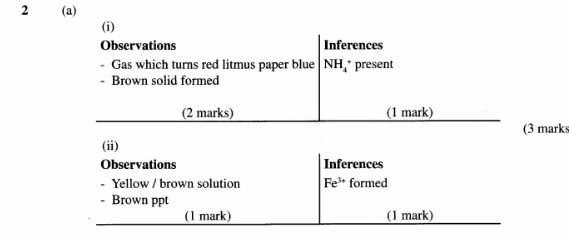
2 (a) You are provided with solid D. Carry out the following tests and write your observations and inferences in the spaces provided.
(i) Place about one half of solid D in a test-tube and heat it strongly. Test any gases produced with both red and blue litmus papers.
Observations (2 marks)
Inferences (1 mark)
(ii) Place the rest of solid D in a boiling tube. Add about 10cm3 of distilled Water. Shake well.
To a 2cm3 portion of the solution, add about 1cm3 of hydrogen peroxide and shake well.
To the resulting mixture, add aqueous sodium hydroxide dropwise until in excess.
Observations (1 mark)
Inferences(1 mark)
(b) You are provided with solution E. Carry out the following tests and write your observations and inferences in the spaces provided.
Divide solution E into two portions.
(i) To one portion of solution E in a test-tube, add 3 drops of barium nitrate. Retain the mixture for use in test (ii) below.
Observations (1 mark)
Inferences (2 marks)
(ii) To the mixture obtained in (i) above, add about 5 cm3 of 2M nitric (V) acid.
Observations (1 mark)
Inferences (1 mark)
(iii) To portion two of solution E in a test-tube, add 2 drops of acidified potassium dichromate (VI) and warm the mixture.
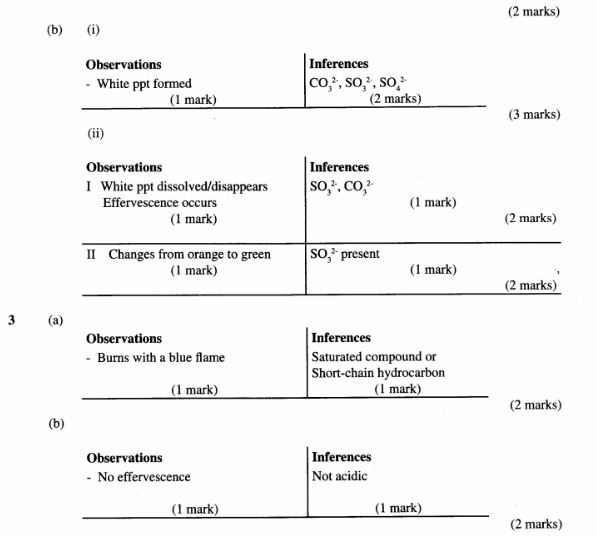
3 You are provided with liquid F. Carry out the following tests and record your observations and inferences in the spaces provided.
(a) Place five drops of liquid F on a clean dry watch glass and ignite it
Observations(1 mark)
Inferences(1 mark)
(b) Place about 2cm3 of liquid F in a clean dry test tube, add all the sodium hydrogen carbonate provided.
Observations (1 mark)
Inference;(1 mark)
(c) Place about 2cm3 of liquid F in a test-tube, add about 1cm3 of acidified potassium dichromate (VI) and warm the mixture.
Observations (1 mark)
Inferences(1 mark)
11.0 CHEMISTRY (233)
11.1 Chemistry Paper 1 (233/1)








Kenya Scholarships for Undergraduate Students » Kenya Scholarships for Postgraduate Students » Undergraduate Scholarships for Kenyan Students » Kenya Undergraduate Scholarships » Full Undergraduate Scholarships for Kenyans » Kenya Postgraduate Scholarships » Scholarships & Grants » Undergraduate Scholarships » Universities in Kenya » Kenya Universities and Colleges Central Placement Service (KUCCPS) » Colleges in Kenya » KASNEB Registration & Results » Secondary Schools Scholarships in Kenya » Undergraduate & Graduate Scholarships for Kenyans
Scholarships for African Students » Undergraduate Scholarships » African Women Scholarships & Grants » Developing Countries Scholarships » Erasmus Mundus Scholarships for Developing Countries » Fellowship Programs » Funding Grants for NGOs » Government Scholarships » LLM Scholarships » MBA Scholarships » PhD and Masters by Research Scholarships » Public Health Scholarships - MPH Scholarships » Refugees Scholarships » Research Grants » Scholarships and Grants
Scholarships in Australia » Scholarships in Belgium » Scholarships in Canada » Scholarships in Germany » Scholarships in Italy » Scholarships in Japan » Scholarships in Korea » Scholarships in Netherlands » Scholarships in UK » Scholarships in USA
KCSE Past Papers Chemistry 2011
"Pdf" Revision Questions Chemistry Form 2 "Pdf" Revision Questions Chemistry Form 3 "Pdf" Revision Questions Chemistry Form 4 "Pdf" Revision Questions Chemistry Form Four "Pdf" Revision Questions Chemistry Form One "Pdf" Revision Questions Chemistry Form Three "Pdf" Revision Questions Chemistry Form Two 1 a a KCSE Past Papers 10th Grade Chemistry Questions and Answers 10th Grade Chemistry Test 11th Ncert Chemistry 12th Class Chemistry Book Free Download 2014 KCSE Marking Schemes 2014 Pdf KCSE Past Papers 2015 2015 Chemistry Essay Questions and Answers Form 4 2016 KCSE Papers 2016 KCSE Prediction Questions 2017 Chemistry Hsc Answers 2017 KCSE Prediction Questions 2018 Chemistry KCSE Leakage 2018 Chemistry KCSE Questions 2018 KCSE Busineness Studies 2018 KCSE Exam 2018 KCSE Leakage 2018 KCSE Prediction Questions 2018 KCSE Questions 2019 Chemistry KCSE Leakage 2019 Chemistry KCSE Questions 2019 KCSE Leakage 2019 KCSE Questions 9th Grade Chemistry Study Guide A a a Chemistry Notes a a a Chemistry Notes! a a a ChemistryNotes! A a KCSE Past Papers A Biblical View of Social Justice A Level Chemistry Biological Molecules Questions A Level Chemistry Exam Questions by Topic A Level Chemistry Notes Edexcel A Level Chemistry Notes Xtremepapers A Level Chemistry Past Papers A Level Chemistry Questions and Answers a Level Chemistry Questions and Answers A Level Chemistry Questions and Answers (Pdf) A Level Chemistry Questions and Answers Pdf A Level Chemistry Questions by Topic Kidney Questions With Markschemes A Level Chemistry Revision A Level Chemistry Revision Edexcel A Level Chemistry Revision Guide A Level Chemistry Revision Notes A Level Chemistry Revision Notes Pdf A Level Chemistry Textbook Pdf A Level Chemistry Year 1 / as Aqa Exam Questions by Topic A Level Edexcel Notes a* Chemistry aa Chemistry Form 3 Questions and Answers Advance KCSE Past Papers Advance-africa.com KCSE Rev Quiz Advantages and Disadvantages. All Chemistry Essays All Chemistry Notes for Senior Two All KCSE Past Papers Chemistry With Making Schemes All Marking Schemes Questions and Answers All Past K.c.s.e Questions With Answers Alliance Mocks 2017 Ap Bio Quizzes Ap Chemistry 1 Textbook Pdf Ap Chemistry Essay Questions and Answers Are Sourced From KNEC. As Level Chemistry Notes Atika Chemistry Notes Atika School Chemistry Notes B/s Book 2 Notes Basic Chemistry Books Pdf basic Chemistry Interview Questions and Answers Pdf Basic Chemistry Interview Questions and Answers Pdf Basic Chemistry Pdf Basic Chemistry Questions and Answers Basic Chemistry Questions and Answers Pdf Bbc Bitesize Chemistry Ks3 Bihar Board Chemistry Objective Answer 2017 Bihar Board Chemistry Objective Answer 2018 Bio Answers Bio Quesions Chemistry 0478 Chemistry 101 Chemistry 12th Chemistry 12th Class Notes Pdf Chemistry 2019 Syllabus Chemistry All KCSE Short Notes Chemistry Answers Chemistry Answers Online Free Chemistry Answers Quizlet Chemistry Bk 2 Notes Chemistry Book 1 Chemistry Book 1 Notes Chemistry Book 2 Chemistry Book 2 Notes Chemistry Book 3 Chemistry Book 3 KLB Chemistry Book 3 Notes Chemistry Book 4 Chemistry Book 4 Notes Chemistry Book 4 Pdf Chemistry Book for Class 11 Chemistry Book Four Chemistry Book Four Notes Chemistry Book One Chemistry Book One Notes Chemistry Book Pdf Free Download Chemistry Book Three Chemistry Book Three Notes Chemistry Book Three Pdf Chemistry Book Two Chemistry Book Two Notes Chemistry Books Form Three Chemistry Bowl Chemistry Study Guide Chemistry Bowl Questions Chemistry Chemistry Bowl Questions Earth Chemistry Chemistry Bowl Questions Math Chemistry Bowl Questions Middle School Chemistry Brekthrough Form Two Notes Chemistry Class 12 Ncert Solutions Chemistry Class 12 Pdf Chemistry Communication Syllabus Chemistry Diagram Software Chemistry Diagrams for Class 11 Chemistry Diagrams for Class 12 Chemistry Diagrams for Class 9 Chemistry Diagrams for Class-10 Chemistry Diagrams in Form 1 Chemistry Diagrams in Form 2 Chemistry Diagrams in Form 3 Chemistry Diagrams in Form 4 Chemistry Diagrams Pdf Chemistry Diagrams to Label Chemistry Essay Questions and Answers Chemistry Essay Questions and Answers 2018 Chemistry Essay Questions and Answers Form 1 Chemistry Essay Questions and Answers Form 2 Chemistry Essay Questions and Answers Form 3 Chemistry Essay Questions and Answers Form 4 Chemistry Essay Questions and Answers Form 4 Pdf Chemistry Essay Questions and Answers Pdf Chemistry Essay Revision Q Chemistry Essays and Answers Chemistry Essays Form One to Form Four Chemistry Essays Form One to Form Three Chemistry Essays KCSE Chemistry Essays Pdf Chemistry Exam 1 Multiple Choice Chemistry Exam 2 Advance Chemistry Exam 2 Test Chemistry Exam 2016 Chemistry Exam Form Four Chemistry Exam Form One Chemistry Exam Form Three Chemistry Exam Form Two Chemistry Exam Practice Test Chemistry Exam Questions Chemistry Exam Questions and Answers Chemistry Exam Questions and Answers Pdf Chemistry Exam Study Guide Chemistry Exams Chemistry Excretion Notes Chemistry Exercise Form 4 With Answers Chemistry Final Exam Answer Key Chemistry Final Exam Answer Key 2016 Chemistry Final Exam Answer Key 2017 Chemistry Final Exam Answers 2018 Chemistry Final Exam Answers 2019 Chemistry Final Exam Questions and Answers Chemistry Fom 1 Notes Chemistry Fom 2 Notes Chemistry Fom 3 Notes Chemistry Fom 4 Notes Chemistry Form 1 Chemistry Form 1 & 2 and Answers Chemistry Form 1 and 2 Essays Chemistry Form 1 and 2 Essays Questions and Answers Chemistry Form 1 Chapter 1 Chemistry Form 1 Diagrams Chemistry Form 1 Exams Chemistry Form 1 Mid Year Exam Chemistry Form 1 Notes Chemistry Form 1 Notes and Questions Chemistry Form 1 Notes Download Chemistry Form 1 Notes Free Download Chemistry Form 1 Notes GCSE Chemistry Form 1 Notes KCSE-kcse Chemistry Form 1 Notes Pdf Chemistry Form 1 Notes Pdf Download Chemistry Form 1 Past Papers Chemistry Form 1 Pdf Chemistry Form 1 Pressure Chemistry Form 1 Question Papers Chemistry Form 1 Questions Chemistry Form 1 Questions and Answers Chemistry Form 1 Questions and Answers Pdf Chemistry Form 1 Quiz Chemistry Form 1 Revision Questions Chemistry Form 1 Summary Notes Chemistry Form 1 Syllabus Chemistry Form 1 Work Chemistry Form 1-4 Notes Chemistry Form 2 Chemistry Form 2 Chapter 1 Chemistry Form 2 Chapter 2 Chemistry Form 2 Diagrams Chemistry Form 2 Exam Paper 2014 Chemistry Form 2 Exams Chemistry Form 2 Notes Chemistry Form 2 Notes and Questions Chemistry Form 2 Notes GCSE Chemistry Form 2 Notes KCSE-kcse Chemistry Form 2 Notes Pdf Chemistry Form 2 Notes Pdf Download Chemistry Form 2 Past Papers Chemistry Form 2 Pdf Chemistry Form 2 Question Papers Chemistry Form 2 Questions Chemistry Form 2 Questions and Answers Chemistry Form 2 Questions and Answers Pdf Chemistry Form 2 Quiz Chemistry Form 2 Revision Notes Chemistry Form 2 Salts Chemistry Form 2 Structure and Bonding Chemistry Form 2 Summary Notes Chemistry Form 2 Syllabus Chemistry Form 2 Work Chemistry Form 3 Chemistry Form 3 and 4 Essays Chemistry Form 3 and 4 Essays Questions and Answers Chemistry Form 3 Chapter 3 Chemistry Form 3 Classification Chemistry Form 3 Diagrams Chemistry Form 3 Ecology Chemistry Form 3 Exams Chemistry Form 3 Notes Chemistry Form 3 Notes and Questions Chemistry Form 3 Notes GCSE Chemistry Form 3 Notes KCSE-kcse Chemistry Form 3 Notes Pdf Chemistry Form 3 Notes Pdf Download Chemistry Form 3 Notes Topic 1 Chemistry Form 3 Past Papers Chemistry Form 3 Pdf Chemistry Form 3 Question Papers Chemistry Form 3 Questions Chemistry Form 3 Questions and Answers Chemistry Form 3 Questions and Answers Pdf Chemistry Form 3 Questions and Answers Term 3 Chemistry Form 3 Questions and Answers+pdf Chemistry Form 3 Quiz Chemistry Form 3 Revision Notes Chemistry Form 3 Revision Questions Chemistry Form 3 Summary Notes Chemistry Form 3 Syllabus Chemistry Form 3 Syllabus Pdf Chemistry Form 3 Topics Chemistry Form 3 Work Chemistry Form 4 Chemistry Form 4 All Chapter Chemistry Form 4 Chapter 1 Conversion of Units Chemistry Form 4 Chapter 1 Exercise Chemistry Form 4 Chapter 1 Exercise and Answers Chemistry Form 4 Chapter 1 Exercise Pdf Chemistry Form 4 Chapter 1 Mind Map Chemistry Form 4 Chapter 2 Chemistry Form 4 Chapter 2 Exercise and Answers Chemistry Form 4 Chapter 2 Exercise Pdf Chemistry Form 4 Chapter 2 Experiment Chemistry Form 4 Chapter 2 Formula Chemistry Form 4 Chapter 2 Mind Map Chemistry Form 4 Chapter 2 Momentum Chemistry Form 4 Chapter 2 Notes Pdf Chemistry Form 4 Chapter 2 Objective Questions and Answers Chemistry Form 4 Chapter 2 Paper 2 Chemistry Form 4 Chapter 2 Slideshare Chemistry Form 4 Chapter 3 Chemistry Form 4 Chapter 3 Questions and Answers Chemistry Form 4 Chapter 4 Chemistry Form 4 Chapter 4 Notes Pdf Chemistry Form 4 Chapter 5 Light Questions and Answers Chemistry Form 4 Chapter 5 Notes Pdf Chemistry Form 4 Diagrams Chemistry Form 4 Exam Paper 1 Chemistry Form 4 Exams Chemistry Form 4 Exercise Chemistry Form 4 Exercise Pdf Chemistry Form 4 Module With Answer Chemistry Form 4 Note Chemistry Form 4 Notes Chemistry Form 4 Notes (Pdf) Chemistry Form 4 Notes All Chapter Pdf Chemistry Form 4 Notes and Questions Chemistry Form 4 Notes Chapter 1 Chemistry Form 4 Notes Chapter 2 Chemistry Form 4 Notes Chapter 3 Chemistry Form 4 Notes Download Chemistry Form 4 Notes Free Download Chemistry Form 4 Notes GCSE Chemistry Form 4 Notes KCSE-kcse Chemistry Form 4 Notes Pdf Chemistry Form 4 Notes Pdf Download Chemistry Form 4 Paper 2 Questions and Answers Chemistry Form 4 Past Papers Chemistry Form 4 Question Papers Chemistry Form 4 Questions Chemistry Form 4 Questions and Answers Chemistry Form 4 Questions and Answers Pdf Chemistry Form 4 Quiz Chemistry Form 4 Revision Notes Chemistry Form 4 Schemes of Work Chemistry Form 4 Summary Notes Chemistry Form 4 Syllabus Chemistry Form 4 Textbook Pdf Chemistry Form 4 Work Chemistry Form 5 Chapter 1 Exercise and Answers Chemistry Form 5 Chapter 1 Notes Pdf Chemistry Form 5 Chapter 2 Notes Pdf Chemistry Form 5 Chapter 2 Slideshare Chemistry Form 5 Chapter 3 Notes Pdf Chemistry Form 5 Notes Pdf Chemistry Form Four Book Chemistry Form Four Notes Chemistry Form Four Notes and Questions Chemistry Form Four Notes GCSE Chemistry Form Four Notes Pdf Chemistry Form Four Past Papers Chemistry Form Four Questions Chemistry Form Four Questions and Answers Chemistry Form Four Questions and Answers Pdf Chemistry Form Four Quiz Chemistry Form Four Study Notes Chemistry Form Four Syllabus Chemistry Form Four Topic 2 Chemistry Form Four Topic 4 Chemistry Form Four Topics Chemistry Form Four Work Chemistry Form One Chemistry Form One Book Chemistry Form One Book Pdf Chemistry Form One Download Topic 1 Upto 3 Chemistry Form One Exam Chemistry Form One Notes Chemistry Form One Notes and Questions Chemistry Form One Notes GCSE Chemistry Form One Notes Pdf Chemistry Form One Pdf Chemistry Form One Questions Chemistry Form One Questions and Answers Chemistry Form One Questions and Answers Pdf Chemistry Form One Questions and Their Answers Chemistry Form One Quiz Chemistry Form One Revision Question Chemistry Form One Schemes of Work Chemistry Form One Study Notes Chemistry Form One Syllabus Chemistry Form One Term Three Test Chemistry Form One to Three Notes Chemistry Form One Work Chemistry Form Three Chemistry Form Three Book Chemistry Form Three Notes Chemistry Form Three Notes and Questions Chemistry Form Three Notes GCSE Chemistry Form Three Questions and Answers Chemistry Form Three Questions and Answers Pdf Chemistry Form Three Quiz Chemistry Form Three Reproduction Chemistry Form Three Reproduction. Chemistry Form Three Study Notes Chemistry Form Three Work Chemistry Form Three-questions and Answers Chemistry Form Two Chemistry Form Two Book Chemistry Form Two Diagrams Chemistry Form Two Notes Chemistry Form Two Notes and Questions Chemistry Form Two Notes GCSE Chemistry Form Two Notes Pdf Chemistry Form Two Notes-pdf Chemistry Form Two Pdf Chemistry Form Two Questions Chemistry Form Two Questions and Answers Chemistry Form Two Questions and Answers Pdf Chemistry Form Two Quiz Chemistry Form Two Study Notes Chemistry Form Two Topics Chemistry Form Two Work Chemistry Form Two,schemes of Work Chemistry Form2 Chemistry Form2 Textbook Chemistry Game Form Four Question End Answers Chemistry Grade 10 Exam Papers Chemistry Hsc Pdf Chemistry Human Reproduction Video Chemistry IGCSE Past Papers Xtremepapers Chemistry K.c.s.e 2017 Chemistry KCSE Chemistry KCSE 2016 Chemistry KCSE 2017 Chemistry KCSE 2017 Paper 1 Chemistry KCSE Past Papers Chemistry KCSE Questions Chemistry KCSE Questions and Answer Chemistry KCSE Quizzes & Answers Chemistry KCSE Revision Chemistry KCSE Revision Notes Chemistry KCSE Setting Questions Form One and Two Chemistry Ksce 2015 Chemistry Last Year K.c.s.e Questions Chemistry Lesson Plan Form Two Chemistry Made Familiar Chemistry Mcq for Class 11 Chemistry Mcq for Class 12 Chemistry Mcq for Competitive Exams Chemistry Mcq for Competitive Exams Pdf Chemistry Mcq for Neet Pdf Chemistry Mcq for Ssc Chemistry Mcq Questions With Answers Chemistry Mcq With Answers Pdf Chemistry Mcqs for Class 12 Pdf Chemistry Mcqs With Answers Pdf Chemistry Mid Familia Form One Chemistry Mock Papers Chemistry Module Form 5 Chemistry Multiple Choice Questions and Answers Cxc Chemistry Multiple Choice Questions and Answers Pdf Chemistry Multiple Choice Questions With Answers Pdf Chemistry Note Chemistry Note Form Two All Chapters Chemistry Notes Chemistry Notes and Guestion and Answear Chemistry Notes and Syllabus Chemistry Notes Class 10 Chemistry Notes for Class 11 Pdf Chemistry Notes for Class 12 Pdf Chemistry Notes for High School Students Chemistry Notes for IGCSE 2014 Chemistry Notes Form 1 Chemistry Notes Form 1 4 Chemistry Notes Form 1 Free Download Chemistry Notes Form 1 KLB Chemistry Notes Form 1 Pdf Chemistry Notes Form 1-4 Chemistry Notes Form 1-4(1) Chemistry Chemistry Notes Form 14 Chemistry Notes Form 2 Chemistry Notes Form 2 KLB Chemistry Notes Form 2 Pdf Chemistry Notes Form 2; Chemistry Notes Chemistry Notes Form 3 Chemistry Notes Form 3 KLB Chemistry Notes Form 3 Pdf Chemistry Notes Form 4 Chemistry Notes Form 4 Chapter 2 Chemistry Notes Form 4 KLB Chemistry Notes Form 4 Pdf Chemistry Notes Form 4-pdf Chemistry Notes Form Four Chemistry Notes Form Four KLB Chemistry Notes Form Four Pdf Chemistry Notes Form One Chemistry Notes Form One KLB Chemistry Notes Form One Pdf Chemistry Notes Form One to Form Four Chemistry Notes Form Three Chemistry Notes Form Three KLB Chemistry Notes Form Three Pdf Chemistry Notes Form Two Chemistry Notes Form Two KLB Chemistry Notes Form Two Pdf Chemistry Notes Form2 Chemistry Notes IGCSE Chemistry Notes Kenya Chemistry Notes on Agroforestry Chemistry Notes Pdf Chemistry Notes: Chemistry Objective Answer Chemistry Objective Answer 2018 Chemistry Objective Questions for Competitive Exams Chemistry Objective Questions for Competitive Exams Pdf Chemistry Oral Exam Questions Chemistry Paper 1 Chemistry Paper 1 2018 Marking Rules Chemistry Paper 1 Notes Chemistry Paper 1 Questions Chemistry Paper 1 Questions and Answers Chemistry Paper 1 Topics Chemistry Paper 1 With Answers Chemistry Paper 2 Chemistry Paper 2 2017 Chemistry Paper 2 2018 Marking Rules Chemistry Paper 2 Questions and Answers Chemistry Paper 2 Questions and Answers Pdf Chemistry Paper 2 Revision Chemistry Paper 2 Topics Chemistry Paper 2018 Chemistry Paper 3 2018 Marking Rules Chemistry Paper 3 Question and Answer Chemistry Paper 3 Question Paper 2014 KCSE Chemistry Paper 3 Question Paper 2015 KCSE Chemistry Paper 3 Question Paper 2016 KCSE Chemistry Paper 3 Question Paper 2017 KCSE Chemistry Paper 3 Question Paper 2018 KCSE Chemistry Paper 3 Questions and Answers Chemistry Paper One Questions and Answers Chemistry Paper One Topics Chemistry Paper Two Qestions With Answers Chemistry Paper1 Chemistry Paper2 Chemistry Paper3 Chemistry Paper4 Chemistry Past Papers Chemistry Past Papers 2017 Chemistry Past Papers a Level Chemistry Past Papers Form 1 Chemistry Past Papers Form 2 Chemistry Past Papers Form 3 Chemistry Past Papers O Level Chemistry Pdf Download Chemistry Pp1 KCSE 2016 Chemistry Practical Book Class 12 Pdf Chemistry Practical Exam Chemistry Practicals Form One Chemistry Practicals Questions and Answers Chemistry Practice Test 9th Grade Chemistry Practice Test Answers Chemistry Practice Test Questions and Answers Chemistry Practice Test Quizlet Chemistry Predicted Questions This Year KCSE Chemistry Preparation Notes Chemistry Pretest High School Pdf Chemistry Question and Answer With Explanation Chemistry Question and Answers 2019 Chemistry Question and Answers 2020 Chemistry Question and Answers 2021 Chemistry Question and Answers 2022 Chemistry Question and Answers Note Chemistry Questions Chemistry Questions and Answers Chemistry Questions and Answers for High School Chemistry Questions and Answers for High Schools Chemistry Questions and Answers for High Schools Pdf Chemistry Questions and Answers for Secondary Schools Chemistry Questions and Answers Form 1 Chemistry Questions and Answers Form 2 Chemistry Questions and Answers Form 3 Chemistry Questions and Answers Form 4 Chemistry Questions and Answers Multiple Choice Chemistry Questions and Answers Notes Chemistry Questions and Answers O Chemistry Questions and Answers Online Chemistry Questions and Answers Pdf Chemistry Questions and Answers Pdf for Class 12 Chemistry Questions and Answers Pdf for Competitive Exams Chemistry Questions and Answers-form 2 Chemistry Questions for High School Chemistry Questions for High School Students With Answers Chemistry Questions for Senior 1 Chemistry Questions for Senior 2 Chemistry Questions for Senior 3 Chemistry Questions for Senior 4 Chemistry Questions for Senior 5 Chemistry Questions for Senior 6 Chemistry Questions for Senior Five Chemistry Questions for Senior Four Chemistry Questions for Senior One Chemistry Questions for Senior Six Chemistry Questions for Senior Three Chemistry Questions for Senior Two Chemistry Questions Form One Chemistry Questions Multiple Choice Chemistry Questions Quizlet Chemistry Questions to Ask Your Teacher Chemistry Quetion and Answer Form Four Chemistry Quetion and Answer Form One Chemistry Quetion and Answer Form Three Chemistry Quetion and Answer Form Two Chemistry Quiz for Class 9 Chemistry Quiz for Class 9 Chemistry Chemistry Quiz Questions and Answers for Class 10 Chemistry Quiz Questions and Answers for Class 10 Pdf Chemistry Quiz Questions and Answers for Class 12 Chemistry Quiz Questions and Answers for Class 9 Chemistry Quiz Questions and Answers for Class 9 Pdf Chemistry Quiz Questions and Answers for High School Chemistry Quiz Questions and Answers Multiple Choice Chemistry Quiz Questions and Answers Pdf Chemistry Quiz Questions for Class 12 Chemistry Quiz Questions for College Students Chemistry Quiz With Answers Chemistry Quiz With Answers Pdf Chemistry Quizlet Chemistry Revision Chemistry Revision a Level Chemistry Revision Chemistry Notes Chemistry Chemistry Revision Exam Chemistry Revision Examination Chemistry Revision Form One Chemistry Revision Notes Chemistry Revision Notes Chemistry Chemistry Revision Notes Form 1 Chemistry Revision Notes Form 2 Chemistry Revision Notes Form 3 Chemistry Revision Notes Form 4 Chemistry Revision Notes IGCSE Chemistry Revision Paper One Chemistry Revision Questions Chemistry Revision Questions and Answers Chemistry Revision Questions and Answers Form 1 Chemistry Revision Questions and Answers Form 2 Chemistry Revision Questions and Answers Form 3 Chemistry Revision Questions and Answers Form 4 Chemistry Revision Questions and Answers Form Four Chemistry Revision Questions and Answers Form One Chemistry Revision Questions and Answers Form Three Chemistry Revision Questions and Answers Form Two Chemistry Revision Questions Form 1 Chemistry Revision Questions Form 2 Chemistry Revision Questions Form 3 Chemistry Revision Questions Form 4 Chemistry Revision Questions Form Four Chemistry Revision Questions Form One Chemistry Revision Questions Form Three Chemistry Revision Questions Form Two Chemistry Revision Quiz Chemistry Revision Test Chemistry Secondary School Revision Chemistry Simple Notes Chemistry Spm Notes Download Chemistry Spm Notes Pdf Chemistry Spm Questions Chemistry Study Form 2 Chemistry Study Guide Chemistry Study Guide Answer Key Chemistry Study Guide Answers Chemistry Study Guide Chemistry Questions and Answers Chemistry Study Guide Ib Chemistry Study Guide Pdf Chemistry Study Guides Chemistry Study Notes Chemistry Study Notes Materials Form 1 Pdf Chemistry Study Notes Materials Form 2 3 Pdf Chemistry Study Notes Materials Form 2 Pdf Chemistry Study Notes Materials Form 3 Pdf Chemistry Study Notes Materials Form 4 Pdf Chemistry Syllabus in Kenya Chemistry Syllabus Pdf Chemistry Test 1 Quizlet Chemistry Test Questions Chemistry Test Questions and Answers Chemistry Test Questions and Answers Pdf Chemistry Topic One Form Four Chemistry Topics Form One Chemistry Unit 1 Quiz Chemistry Vol 3 Chemistry | Revision Chemistry Chemistry,form 4 Chemistry.form Four.topic Three ChemistryExam Form Three ChemistryModule Form 5 ChemistryNotes ChemistryNotes for Class 11 Pdf ChemistryNotes for Class 12 Pdf ChemistryNotes Form 1 ChemistryNotes Form 1 Free Download ChemistryNotes Form 2 ChemistryNotes Form 3 ChemistryNotes Form 3 Pdf ChemistryNotes IGCSE ChemistryNotes Pdf ChemistryPast Papers ChemistryQuestions and Answers Pdf ChemistrySimple Notes ChemistrySpm Notes Download ChemistrySpm Notes Pdf ChemistrySpm Questions ChemistryStudy Guide Answers ChemistryStudy Guide Pdf ChemistryStudy Guides Blologytextpapers Bridge Chemistry Business Past KCSE Past Papers Business Studies Form 3 Notes Pdf Business Studies Form 4 Notes Pdf C R E Form One KLB C R E Form One Oli Topic C.r.e Form 1 Notes Kenya C.r.e Form 2 Notes Kenya C.r.e Form 3 Notes C.r.e Form 3 Notes Kenya C.r.e Form 3 Pdf C.r.e Form 4 Notes Kenya C.r.e Form One Notes Pdf C.r.e Notes Form 1 C.r.e Revision Notes C.r.e Short Notes Cambridge IGCSE Chemistry Cambridge IGCSE Chemistry 3rd Edition Cambridge IGCSE Chemistry 3rd Edition Plus Cd South Asia Edition Cambridge IGCSE Chemistry Answers Cambridge IGCSE Chemistry Coursebook Pdf Download Cambridge IGCSE Chemistry Practical Workbook Cambridge IGCSE Chemistry Revision Guide Pdf Cambridge IGCSE Chemistry Study and Revision Guide 2nd Edition Pdf Cambridge IGCSE Chemistry Study and Revision Guide Pdf Cambridge IGCSE Chemistry Workbook Free Download Cambridge IGCSE Chemistry Workbook Pdf Cambridge IGCSE® Chemistry Coursebook Caucasian Chalk Circle Essay Questions Chapter 1 Introduction to Chemistry Chapter 1 Introduction to Chemistry Studies Cie a Level Chemistry Notes 2016 Cie a Level Chemistry Notes Pdf Cie Past Papers Class 10 Chemistry Chapter 1 Mcqs Class 8 Chemistry Notes KCSE-kcse College Chemistry Notes College Chemistry Practice Test College Chemistry Quiz College Chemistry Quiz Chapter 1 College Chemistry Quizlet College Chemistry Study Guide College Chemistry Study Guide Pdf College Chemistry Test Questions and Answers College Chemistry Volume 3 Pdf College ChemistryNotes Complete Chemistry for Cambridge IGCSE Complete Chemistry for Cambridge IGCSE Revision Guide Pdf County Mocks 2017 Cse Past Papers Chemistry 2017 Dl Chemistry Form 3 Pdf Kusoma Download Chemistry Form 1 Download Chemistry Form 2 Download Chemistry Form 2 Notes Download Chemistry Form 3 Download Chemistry Form 3 Notes Download Chemistry Form 4 Download Chemistry Form Four Download Chemistry Form One Download Chemistry Form Three Download Chemistry Form Two Download Chemistry Notes Form 3 Download Chemistry Notes Form One Download ChemistryNotes Form 3 Download Form Three Chemistry Notes Download Free KCSE Past Papers Chemistry Download Free KCSE Past Papers From KNEC. Download KCSE Past Papers With Answers Download KCSE Revision Notes Download KLB Chemistry Book 2 Download KLB Chemistry Book 3 Download KLB Chemistry Book 4 Download Notes of Chemistry Downloads | Chemistry | Form Four Exams | Exams Downloads | Chemistry | Form One Exams | Exams Downloads | Chemistry | Form Three Exams | Exams Downloads | Chemistry | Form Two Exams | Exams Downloads | KCSE Papers and Marking Schemes | Dvance KCSE Past Papers Easy Chemistry Questions Edexcel a Level Chemistry B Edexcel a Level Chemistry Notes Pdf Edexcel a Level Chemistry Salters Nuffield Edexcel A2 Chemistry Notes Edexcel as Chemistry Revision Guide Pdf Edexcel Chemistry A2 Revision Notes Pdf Edexcel Chemistry Unit 2 Revision Notes Edexcel GCSE Chemistry Revision Guide Pdf Edexcel IGCSE Chemistry Past Papers Edexcel IGCSE Chemistry Revision Guide Free Pdf Download Edexcel IGCSE Chemistry Revision Guide Pdf Edexcel IGCSE Chemistry Revision Guide Pdf Download Electronics Form Four Notes Energy Questions Chemistry Bowl Essay Questions and Answers KCSE Chemistry Notes Essay Questions and Answers on Betrayal in the City Essay Questions Based on Betrayal in the City Evolving World Chemistry Book 1 Pdf Evolving World Chemistry Book 4 Notes Evolving World Chemistry Book Form 1 Evolving World-history Book 3 Exam Notes for Chemistry 101 Exams KCSE Chemistry Paper 1 Questions and Answers F3 Chemistry Test Paper Find Download KCSE Past Papers With Answers Find KCSE Chemistry Essay Questions and Answers Form 1 Chemistry Exam Form 1 Chemistry Notes Form 1 Chemistry Questions and Answers Form 1 Chemistry Questions and Answers Pdf Form 1 Chemistry Revision Notes Form 1 Chemistry Summurized Revision Pdf Form 1 Chemistry Syllabus Form 1 Chemistry Test Paper Pdf Form 1 Chemistry Topics Form 1 ChemistryNotes Form 1 ChemistryQuestions and Answers Form 1 ChemistryRevision Notes Form 1 ChemistrySyllabus Form 1 ChemistryTest Paper Pdf Form 1 Past Papers Form 1 Past Papers With Answers Form 1 Revision Papers Form 1 Subjects in Kenya Form 2 Chemistry Exam Form 2 Chemistry Exam Paper Form 2 Chemistry Exam Paper 2016 Form 2 Chemistry Exam Paper Free Download Form 2 Chemistry Exam Paper With Answer Form 2 Chemistry Final Year Exam Paper 2 Form 2 Chemistry Notes Form 2 Chemistry Notes and Revision Questions Form 2 Chemistry Notes Pdf Form 2 Chemistry Past Papers Form 2 Chemistry Questions Form 2 Chemistry Questions and Answers Form 2 Chemistry Questions and Answers > Form 2 Chemistry Questions and Answers Pdf Form 2 Chemistry Revision Notes Form 2 Chemistry Short Notes Form 2 Chemistry Syllabus Form 2 ChemistryExam Paper Form 2 ChemistryExam Paper Free Download Form 2 ChemistryExam Paper With Answer Form 2 ChemistryFinal Year Exam Paper 2 Form 2 ChemistryPast Papers Form 2 ChemistryRevision Notes Form 2 ChemistryShort Notes Form 2 ChemistrySyllabus Form 2 Revision Papers Form 2 Subjects in Kenya Form 3 Chemistry Book Form 3 Chemistry Exam Form 3 Chemistry Exam Paper Form 3 Chemistry Notes Form 3 Chemistry Past Papers Form 3 Chemistry Questions Form 3 Chemistry Questions and Answers Form 3 Chemistry Questions and Answers Pdf Form 3 Chemistry Revision Notes Form 3 Chemistry Syllabus Form 3 ChemistryExam Paper Form 3 ChemistryNotes Form 3 ChemistryPast Papers Form 3 ChemistryQuestions Form 3 ChemistryQuestions and Answers Pdf Form 3 ChemistryRevision Notes Form 3 ChemistrySyllabus Form 3 C.r.e Form 3 Notes of Chemistry Topic on Fish Form 3 Past Papers Form 3 Revision Papers Form 3 Subjects in Kenya Form 4 Chemistry Exam Form 4 Chemistry Notes Form 4 Chemistry Notes Pdf Form 4 Chemistry Questions and Answers Form 4 Chemistry Questions and Answers Pdf Form 4 Chemistry Revision Notes Form 4 Chemistry Syllabus Form 4 Chemistry Topics Form 4 ChemistryNotes Form 4 ChemistryRevision Notes Form 4 ChemistrySyllabus Form 4 ChemistryTopics Form 4 Exam Papers Form 4 Revision Papers Form 4 Subjects in Kenya Form 5 Chemistry Topics Form 5 ChemistryTopics Form Five Chemistry Notes Form Five ChemistryNotes Form Four Chemistry Book Form Four Chemistry Notes Form Four Chemistry Notes Pdf Form Four Chemistry Questions and Answers Form Four Chemistry Questions and Answers Pdf Form Four Chemistry Revision Questions Form Four Chemistry Syllabus Form Four Chemistry Topics Form Four ChemistryNotes Form Four ChemistryQuestions and Answers Form Four ChemistryQuestions and Answers Pdf Form Four ChemistryTopics Form Four Notes Form Four Revision Papers Form Four Subjects in Kenya Form One Chemistry Book Form One Chemistry Examination Form One Chemistry First Topic Form One Chemistry Lesson Plan Form One Chemistry Notes Pdf Form One Chemistry Past Papers Pdf Form One Chemistry Questions Form One Chemistry Questions and Answers Form One Chemistry Questions and Answers Pdf Form One Chemistry Revision Questions Form One Chemistry Short Notes Form One Chemistry Syllabus Form One Chemistry Topics Form One ChemistryExamination Form One ChemistryPast Papers Pdf Form One ChemistryQuestions and Answers Form One ChemistryQuestions and Answers Pdf Form One ChemistryTopics Form One Exams Form One Notes of Chemistry Form One Past Papers Form One Subjects in Kenya Form One Term One Chemistry Exam Form One Term One ChemistryExam Form Three Chemistry Book Form Three Chemistry Book Pdf Form Three Chemistry Notes Form Three Chemistry Notes Pdf Form Three Chemistry Questions and Answers Form Three Chemistry Questions and Answers Pdf Form Three Chemistry Revision Questions Form Three Chemistry Syllabus Form Three Chemistry Topics Form Three ChemistryNotes Form Three ChemistryNotes Pdf Form Three ChemistryQuestions and Answers Form Three ChemistryQuestions and Answers Pdf Form Three ChemistryTopics Form Three Subjects in Kenya Form Two Chemistry Book Form Two Chemistry Cat Form Two Chemistry Examination Form Two Chemistry Notes Form Two Chemistry Notes Pdf Form Two Chemistry Past Papers Form Two Chemistry Questions and Answers Form Two Chemistry Questions and Answers Pdf Form Two Chemistry Revision Questions Form Two Chemistry Syllabus Form Two Chemistry Topics Form Two ChemistryNotes Form Two ChemistryNotes Pdf Form Two ChemistryQuestions and Answers Form Two ChemistryQuestions and Answers Pdf Form Two ChemistrySyllabus Form Two ChemistryTopics Form Two Notes Form Two Subjects in Kenya Free a-level Chemistry Revision App | Pass Your Chemistry Exams Free Chemistry Form 1 Notes Free Chemistry Notes Form 1 Free Chemistry Notes Pdf Free ChemistryNotes Pdf Free College Chemistry Practice Test Free Form1,form2,form3 Past Papers Free KCSE Past Papers Free KCSE Mocks 2015 Free KCSE Past Papers 2014 Free KCSE Past Papers KCSE Past Free KCSE Past Papers Kenya, Free KCSE Past Papers With Answers Free KCSE Questions and Answers on Chemistry Free KCSE Revision Notes Free Marking Schemes Free Mocks Online KCSE Answers Past Exams Question Papers Free Revision Papers From Three Notes Topic One KLB Fun Chemistry Questions Funny Chemistry Questions Funny Chemistry Questions and Answers Funny Chemistry Questions to Ask Funny Chemistry Quotes GCSE Chemistry Exam Questions and Answers GCSE Chemistry Past Papers GCSE Chemistry Revision GCSE Chemistry Revision Notes GCSE Chemistry Revision Notes Pdf GCSE Chemistry Revision Notes Pdf 9-1 GCSE Chemistry Revision Questions and Answers GCSE Chemistry Textbook Pdf GCSE Chemistry Topics Pass My Exams: Easy Exam Revision Notes General Chemistry Notes Pdf General Chemistry Practice Test With Answers General Chemistry Quiz General Chemistry Quiz Pdf General Chemistry Test Questions and Answers General Chemistry Test Questions and Answers Pdf General Knowledge in Chemistry Human Body Good Chemistry Questions to Ask GRE Chemistry Practice Test GRE Chemistry Subject Test Pdf Handbook of Chemistry Pdf Free Download Hard Chemistry Questions Hard Chemistry Questions and Answers Hard Chemistry Questions to Ask Your Teacher Hard Chemistry Quiz Questions Hard Form 3 Chemistry Question High School Chemistry Final Exam Doc High School Chemistry Final Exam Pdf High School Chemistry Final Exam Questions High School Chemistry Final Exam Questions and Answers High School Chemistry Notes High School Chemistry Practice Test High School Chemistry Pretest With Answers High School Chemistry Questions and Answers Pdf High School Chemistry Study Guide High School Chemistry Test Questions and Answers Pdf High School ChemistryNotes High School ChemistryStudy Guide How to Answer KCSE Chemistry Question How to Motivate a Form 4 Student How to Motivate a KCSE Candidate How to Motivate a KCSE Student How to Pass Chemistry Questions & Answers Form 1&2 | Text Book How to Revise Chemistry How to Revise Effectively for KCSE How to Study Chemistry: 5 Study Techniques to Master Chemistry Hsc Chemistry 2018 Hsc Chemistry 2019 Https://www.knec.ac.ke/ Www.knec-portal.ac.ke/ KNEC Portal: Ial Chemistry Notes Ib Chemistry Cold War Notes Ib Chemistry Notes Ib Chemistry Notes Pdf Ib Chemistry of the Americas Notes Ib Chemistry of the Americas Study Guide Ib Chemistry Paper 2 Study Guide Ib Chemistry Question Bank by Topic Ib Chemistry Study Guide Pdf Ict Notes Form 1 IGCSE Chemistry Alternative to Practical Revision IGCSE Chemistry Alternative to Practical Revision Notes IGCSE Chemistry Book IGCSE Chemistry Book Pdf Download IGCSE Chemistry Notes IGCSE Chemistry Notes 2017 Pdf IGCSE Chemistry Notes Edexcel IGCSE Chemistry Paper 2 Notes IGCSE Chemistry Paper 6 Notes IGCSE Chemistry Past Papers IGCSE Chemistry Past Papers 2014 IGCSE Chemistry Past Papers 2017 IGCSE Chemistry Pdf IGCSE Chemistry Pre Release Material 2018 IGCSE Chemistry Resources IGCSE Chemistry Revision Guide IGCSE Chemistry Revision Guide Free Download IGCSE Chemistry Revision Guide Pdf Download IGCSE Chemistry Revision Notes Pdf IGCSE Chemistry Revision Worksheets IGCSE Chemistry Workbook Pdf IGCSE Chemistry Znotes IGCSE ChemistryPast Papers IGCSE Notes Chemistry Importance of Agroforestry Inorganic Chemistry Multiple Choice Questions With Answers Pdf Inorganic Chemistry Questions and Answers Pdf Interesting Chemistry Questions Interesting Chemistry Questions and Answers Interesting Questions to Ask About Chemistry Intro to Chemistry Quiz Introduction of Chemistry Form One Introduction to Chemistry Introduction to Chemistry Notes Introduction to Chemistry Pdf Introduction to ChemistryNotes Is Agroforestry Sustainable? K.c.s.e Answers Chemistry Paper One 2018 K.c.s.e Chemistry 2017 K.c.s.e Chemistry 2018 K.c.s.e Chemistry Paper 1 2017 K.c.s.e Mocks 2018 K.c.s.e Papers 2015 K.c.s.e Papers 2016 K.c.s.e Past Papers 2014 K.c.s.e.Chemistry Paper 2 Year 2018 K.c.s.e.results 2018 for Busia County K.l.b Chemistry Form 3 K.l.b Chemistry Notes K.l.b ChemistryNotes Kasneb Past Papers for Colleges Chemistry Past Papers KCSE 2010 Marking Scheme KCSE 2010 Past Papers KCSE 2011 Chemistry Paper 1 KCSE 2011 Marking Scheme KCSE 2012 Chemistry Paper 2 Marking Scheme KCSE 2012 Marking Schemes KCSE 2013 Chemistry Paper 1 KCSE 2013 Marking Scheme KCSE 2013 Marking Scheme Pdf KCSE 2014 KCSE 2015 Chemistry Paper 2 KCSE 2015 Chemistry Paper 3 KCSE 2015 Marking Scheme KCSE 2015 Past Papers KCSE 2016 Chemistry Paper 1 KCSE 2016 Chemistry Paper 2 KCSE 2017 Chemistry Paper 1 KCSE 2017 Chemistry Paper 2 KCSE 2017 Hostory Papers With Answers.com KCSE 2017 Marking Scheme KCSE 2017 Papers KCSE 2017 Papers and Marking Scheme KCSE 2017 Papers Pdf KCSE 2017 Past Papers KCSE 2017 Prediction Pdf KCSE 2018 Chemistry and Answers KCSE 2018 Chemistry Prediction KCSE 2018 Leakage KCSE 2018 Marking Scheme KCSE 2018 Papers KCSE 2018 Prediction Pdf KCSE 2018 Predictions KCSE 2018 Questions KCSE 2018 Questions and Answers KCSE 2019 Leakage Chemistry KCSE 2019 Marking Scheme KCSE 2019 Questions KCSE 2019 Questions and Answers KCSE 2020 Questions KCSE 2020 Questions and Answers KCSE Answers KCSE Answers Past Exams Question Papers Downloads | KCSE Chemistry 2011 KCSE Chemistry 2016 KCSE Chemistry Diagramsbiology Revision Tips KCSE Chemistry Essay Questions and Answers KCSE Chemistry Essay Questions and Answers Pdf KCSE Chemistry Essays KCSE Chemistry Essays Pdf KCSE Chemistry Marking Schemes KCSE Chemistry Notes KCSE Chemistry Notes Pdf KCSE Chemistry Notes, Syllabus, Questions, Answers KCSE Chemistry Paper 1 KCSE Chemistry Paper 1 2011 KCSE Chemistry Paper 1 2012 KCSE Chemistry Paper 1 2013 KCSE Chemistry Paper 1 2015 KCSE Chemistry Paper 1 2016 KCSE Chemistry Paper 1 2017 KCSE Chemistry Paper 1 2017 Pdf KCSE Chemistry Paper 1 Questions and Answers KCSE Chemistry Paper 2 KCSE Chemistry Paper 2 2012 KCSE Chemistry Paper 2 2012 KCSE Chemistry Paper 2 2015 KCSE Chemistry Paper 2 2013 KCSE Chemistry Paper 2 2014 KCSE Chemistry Paper 2 2015 KCSE Chemistry Paper 2 2016 KCSE Chemistry Paper 2 2017 KCSE Chemistry Paper 3 KCSE Chemistry Paper 3 2012 KCSE Chemistry Paper 3 2016 KCSE Chemistry Paper 3 2017 KCSE Chemistry Paper 3 Past Papers KCSE Chemistry Past Papers KCSE Chemistry Past Papers and Answers KCSE Chemistry Past Papers Pdf KCSE Chemistry Practical KCSE Chemistry Practical 2015 KCSE Chemistry Practical 2016 KCSE Chemistry Practical Past Papers KCSE Chemistry Practicals KCSE Chemistry Practicals KCSE Chemistry Paper 1 KCSE Chemistry Question and Answer KCSE Chemistry Questions and Answers KCSE Chemistry Questions and Answers Ap Chemistry KCSE Chemistry Revision KCSE Chemistry Revision Notes KCSE Chemistry Revision Papers KCSE Chemistry Revision Questions KCSE Chemistry Revision Questions and Answers KCSE Chemistry Syllabus KCSE ChemistryNotes KCSE ChemistryPaper 1 KCSE ChemistryPaper 2 KCSE ChemistryPaper 2 Pdf KCSE ChemistrySyllabus KCSE Business Paper 1 2016 KCSE Business Past Papers KCSE Business Studies Past Papers KCSE Essay Questions in Betrayal in the City KCSE Essays KCSE Exam Papers 2018 KCSE Exam Papers Answers KCSE Form 1 Chemistry Revision KCSE Form 2 Chemistry Revision KCSE Form 3 Chemistry Revision KCSE Form 4 Chemistry Revision KCSE Form Four Chemistry Revision KCSE Form One Chemistry Revision KCSE Form Three Chemistry Revision KCSE Form Two Chemistry Revision KCSE KCSE Past Papers KNEC KCSE Leakage KCSE Leakage Chemistry KCSE Made Familiar Chemistry KCSE Made Familiar Chemistry Pdf KCSE Marking Scheme 2016 KCSE Marking Schemes KCSE Marking Schemes 2017 KCSE Marking Schemes Pdf KCSE Mock Exams KCSE Mock Papers 2015 KCSE Mock Papers 2017 KCSE Mock Papers 2018 KCSE Mock Papers Pdf KCSE Mock Papers Pdf 2018 KCSE Mock Papers Pdf KCSE Past Papers KCSE Mocks 2017 KCSE Mocks 2018 KCSE Notes KCSE Online Notes KCSE Online Past Papers KCSE Online Registration KCSE Papers 2015 KCSE Papers and Marking Schemes | Exams KCSE Past Papers KCSE Past Papers 2007 KCSE Past Papers 2009 KCSE Past Papers 2010 KCSE Past Papers 2011 KCSE Past Papers 2011 Pdf KCSE Past Papers 2012 KCSE Past Papers 2013 KCSE Past Papers 2013knec KCSE Past Papers 2014 KCSE Past Papers 2014 Pdf KCSE Past Papers 2015 KCSE Past Papers 2015 Marking Schemes KCSE Past Papers 2015 Pdf KCSE Past Papers 2016 KCSE Past Papers 2016 Pdf KCSE Past Papers 2017 KCSE Past Papers 2017 Pdf KCSE Past Papers 2018 KCSE Past Papers Chemistry KCSE Past Papers Chemistry and Answers KCSE Past Papers Chemistry Pdf KCSE Past Papers Chemistry With Answers KCSE Past Papers Chemistryand Answers KCSE Past Papers Business Studies and Answers KCSE Past Papers KCSE and Answers KCSE Past Papers KCSE and Answers Free Mocks Online KCSE Past Papers Marking Scheme KCSE Past Papers Pdf Download KCSE Past Papers Pdf Download KCSE 2013 KCSE Past Papers With Answers KCSE Past Papers Woodwork and Answers KCSE Prediction 2017 KCSE Prediction 2018 KCSE Prediction 2018 Pdf KCSE Prediction Papers 2018 KCSE Prediction Questions KCSE Prediction Questions 2018 KCSE Prediction Questions and Answers KCSE Questions KCSE Questions and Answers KCSE Questions and Answers. KCSE Questions on Chemistry KCSE Results, Online Registration, KCSE Result Slip. KCSE Revision KCSE Revision Notes KCSE Revision Notes Chemistry KCSE Revision Notes Pdf KCSE Revision Papers KCSE Revision Papers 2014 KCSE Revision Papers With Answers KCSE Revision Question for Chemistry KCSE Revision Questions KCSE Revision Questions and Answers KCSE Revision | Secondary School | Text Books | Text Book Centre KCSE Syllabus Pdf KCSE Trial 2017 KCSE Trial Exams 2017 Kenya Secondary School Chemistry Syllabus Kenya Secondary School Chemistry Syllabus Pdf Kenya Secondary School ChemistrySyllabus Pdf Kenya Secondary School Syllabus Pdf Kenya-kcse-christian Religious Education Syllabus Kenyaplex KCSE Past Papers Kenyaplex Past Papers for Secondary KLB Chemistry Book 1 Download KLB Chemistry Book 1 Notes KLB Chemistry Book 1 Pdf KLB Chemistry Book 2 KLB Chemistry Book 2 Notes KLB Chemistry Book 2 Notes Pdf KLB Chemistry Book 2 Pdf KLB Chemistry Book 3 Notes KLB Chemistry Book 3 Pdf KLB Chemistry Book 3 Pdf Download KLB Chemistry Book 4 Notes KLB Chemistry Book 4 Pdf KLB Chemistry Book 4 Pdf Download KLB Chemistry Book 4 Topics KLB Chemistry Book One KLB Chemistry Form 1 KLB Chemistry Form 1 Notes KLB Chemistry Form 1 Pdf KLB Chemistry Form 2 KLB Chemistry Form 2 Book KLB Chemistry Form 2 Notes KLB Chemistry Form 2 Pdf KLB Chemistry Form 2 Pdf Download KLB Chemistry Form 2 Schemes of Work KLB Chemistry Form 3 KLB Chemistry Form 3 Notes KLB Chemistry Form 3 Notes Pdf KLB Chemistry Form 3 Pdf KLB Chemistry Form 3 Pdf Download KLB Chemistry Form 4 KLB Chemistry Form 4 Notes KLB Chemistry Form 4 Pdf KLB Chemistry Form Four KLB Chemistry Form Four Notes KLB Chemistry Form One KLB Chemistry Form One Notes KLB Chemistry Form Three KLB Chemistry Form Three Notes KLB Chemistry Form Two KLB Chemistry Form Two Notes KLB Chemistry Notes KLB Chemistry Notes Form 4 KLB Chemistry Pdf KLB ChemistryNotes KLB ChemistryNotes Form 4 KLB ChemistryPdf KNEC Chemistry Syllabus KNEC Examiners Portal KNEC Website KNEC Ict Past Papers KNEC Past Papers for Colleges KNEC Past Papers Free Download KNEC Past Papers Free Downloads KNEC Past Papers Pdf KNEC Portal Confirmation KNEC Portal KCSE Results KNEC Portal KNEC Past Papers for Colleges Kasneb Past Papers KNEC Revision Papers KNEC Technical Exams Past Papers Kusoma Chemistry Notes Kusoma Chemistry Notes Pdf Kusoma Notes Chemistry Kusoma.co.ke Kusoma.com Past Papers Learner Guide for Cambridge IGCSE Chemistry Longhorn Chemistry Book 3 Pdf Made Familiar Chemistry Made Familiar Chemistry Pdf Made Familiar Chemistry Questions Maktaba Tetea Notes Marking Scheme KCSE Chemistry Past Papers Math Form2 Note Mcqs About Gaseous Exchange Middle School Chemistry Bowl Chemistry Questions Mock Past Papers 2017 Mock Past Papers With Answers Mokasa Mock 2017 More Than 1800 Chemistry Questions and Answers to Help You Study Multiple Choice Questions on Chemistry Necta Chemistry Past Papers Necta Chemistry Practicals Necta ChemistryPast Papers Necta ChemistryPracticals Necta Form Four Past Papers Necta Past Papers Form 4 Necta Past Papers Form 4 2016 Necta Past Papers Form Six Necta Past Papers Form Two Necta Questions and Answers Necta Review Questions Notes Chemistry Form 1 Notes Chemistry Form 2 Notes Chemistry Form 3 Notes Chemistry Form 3 Notes Pdf Notes Chemistry Form 3 Syllabus Notes Chemistry Form 4 Syllabus Notes on Chemistry Studies Notes Za Chemistry 4m 2 Notes Za Chemistry Form One Notes Za Chemistry Form Three O Level Chemistry Practical Experiments O Level Chemistry Questions and Answers Pdf Orm Three Chemistry Notes Page Navigation Papacambridge Chemistry IGCSE Papers KNEC KCSE Online Past Papers KNEC KCSE Results Past Papers Past KCSE Papers Past Paper Questions by Topic Chemistry Past Papers 2014 Past Papers in Kenya Pdf Chemistry Form 3 Pdf Chemistry Notes Pdf Chemistry Notes Form 1 Pdf Chemistry Notes Form 2 Pdf Chemistry Notes Form 3 Pdf Chemistry Notes Form 4 Pdf Chemistry Notes Form Four Pdf Chemistry Notes Form One Pdf Chemistry Notes Form Three Pdf Chemistry Notes Form Two Pdf Form 1 Chemistry Questions and Answers Pdf Form 2 Chemistry Questions and Answers Pdf Form 3 Chemistry Questions and Answers Pdf Form 4 Chemistry Questions and Answers Pdf Form Four Chemistry Questions and Answers Pdf Form One Chemistry Questions and Answers Pdf Form Three Chemistry Questions and Answers Pdf Form Two Chemistry Questions and Answers Pdf Free KCSE Past Papers and Marking Schemes Pdf" Revision Questions Chemistry Form 1 Practical Chemistry Experiments Pdf Practical Chemistry Question and Answer Pdf Pre Mocks 2018 Preliminary Chemistry Primary and Secondary Tillage Implements Ppt Pte KNEC Past Papers Questions and Answers Pdf Chemistry Form 1 Questions and Answers Pdf Chemistry Form 2 Questions and Answers Pdf Chemistry Form 3 Questions and Answers Pdf Chemistry Form 4 Questions and Answers Pdf Chemistry Form Four Questions and Answers Pdf Chemistry Form One Questions and Answers Pdf Chemistry Form Three Questions and Answers Pdf Chemistry Form Two Questions Based to Introduction to Chemistry Questions on Gaseous Exchange in Humans Questions on Introduction to Chemistry Questions to Ask in Chemistry Class Questions to Confuse Your Chemistry Teacher Quizlet Chemistry Test Quizlet Test Questions Qustions in Chemistry and Answers Revision Revision Chemistry Notes and Questions? Revision Quiz for Chemistry for Form Three S.1 Chemistry Questions S.2 Chemistry Questions S.3 Chemistry Questions S.4 Chemistry Questions Sample Essays on Betrayal in the City School Chemistry Notes Secondary Chemistry Notes Secondary Chemistry Notes Pdf Secondary ChemistryNotes Pdf Senior 1 Chemistry Notes Senior 2 Chemistry Notes Senior 3 Chemistry Notes Senior 4 Chemistry Notes Senior 5 Chemistry Notes Senior 6 Chemistry Notes Senior Five Chemistry Notes Senior Four Chemistry Notes Senior One Chemistry Notes Senior Six Chemistry Notes Senior Three Chemistry Notes Senior Two Chemistry Notes Simple Scientific Questions Smart Questions to Ask a Chemistry Teacher Snab Chemistry Revision Notes Southwest Mock Paper 2 2016 Chemistry Only Spm Chemistry Revision Notes Spm Notes Success Chemistry Spm Pdf Success ChemistrySpm Pdf Summary of Chemistry Form 3 Tahossa Past Papers To Motivate a Form 4 KCSE Student To Motivate a Form 4 Student Topical Revision Material Tricky Chemistry Questions and Answers Tricky Chemistry Questions for Adults Tricky Chemistry Questions With Answers Tricky Chemistry Quiz Questions Two Chemistry Revision Questions University Chemistry Volume 3 Openstax University Chemistry Volume 3 Pdf University Chemistry Volume 4 Pdf Ur Revision Guide IGCSE Chemistry What Are the Types of Gametes Working of Excretory System Www.Chemistry Form One Notes.com Www.Chemistry From One KLB.com Www.form 1 Chemistry.com Www.form 2 Chemistry.com Www.form 3 Chemistry.com Www.form 4 Chemistry.com Www.form Four Chemistry.com Www.form One Chemistry.com Www.form Three Chemistry.com Www.form Two Chemistry.com Www.kusoma Notes Www.kusoma Revision Materials Www.kusoma.co.ke Chemistry Notes Xtremepapers IGCSE Chemistry Year 11 Chemistry Z Notes Chemistry IGCSE Znotes as Chemistry Acids and Bases Chem Revision Questions Chemistry Notes Combine Form 1, 2, 3 and 4 Chemistry Notes Form 3 A Level Chemistry Questions and Answers Pdf All Chemistry Questions and Answers Pdf,ppt Chemistry Exam Questions and Answers Pdf Chemistry Multiple Choice Questions and Answers Pdf Chemistry Questions and Answers for High Schools Pdf Chemistry Notes Form 1-4 Pdf Free High School Notes Kenya Free Kcse Revision Notes General Chemistry Test Questions and Answers Pdf High School Chemistry Multiple Choice Questions and Answers Pdf Kcse Chemistry Revision Notes Pdf Kcse Revision Books Pdf Kcse Revision Notes Pdf Kenya Secondary School Notes Pdf Notes of Form 123 and 4 All Subject Chemistry Notes Form 1 Free Download Advice to KCSE Candidates Best Revision Books for KCSE How to Pass an Exam Successfully How to Pass KCSE 2018 How to Pass KCSE 2019 How to Pass KCSE Chemistry Paper How to Pass KCSE Chemistry How to Pass KCSE Chemistry How to Pass KCSE 2019 K.c.s.e Chemistry Paper 1 2017 KCSE 2019 Prediction KCSE Prediction 2019 KCSE Revision Tips KCSE Chemistry Paper 1 2018 KCSE Chemistry Paper 2 2018 KCSE Chemistry Past Papers Pdf KCSE Past Papers 2012 KCSE Past Papers 2017 Pdf KCSE Past Papers 2018 KCSE Past Papers of Chemistry Pp2 KCSE Chemistry Past Papers Pdf Chemistry KCSE Papers With Their Marking Schemes Chemistry Paper 1 and Answers KCSE 2017 Papers and Marking Scheme KCSE 2019 Papers and Marking Scheme KCSE Chemistry Paper 1 2018 KCSE Chemistry Paper 1 2019 KCSE Chemistry Paper 2 2017 KCSE Chemistry Paper 2 2018 KCSE Chemistry Paper 2 2019 KCSE Chemistry Past Papers and Answers KCSE Marking Schemes Pdf KCSE Past Papers 2015 Marking Schemes KCSE Past Papers 2019 Marking Schemes Chemistry KCSE Papers With Their Marking Schemes Chemistry Paper 1 and Answers KCSE 2017 Papers and Marking Scheme KCSE 2019 Papers and Marking Scheme KCSE Chemistry Paper 1 2018 KCSE Chemistry Paper 1 2019 KCSE Chemistry Paper 1 2019 Past Papers KCSE Chemistry Paper 2 2017 KCSE Chemistry Paper 2 2018 KCSE Chemistry Paper 2 2019 KCSE Chemistry Paper 2 2019 Past Papers KCSE Chemistry Paper 3 2019 Past Papers KCSE Chemistry Past Papers and Answers KCSE Marking Schemes Pdf KCSE Past Papers 2015 Marking Schemes KCSE Past Papers 2019 Marking Schemes KCSE Past Papers Chemistry Paper 1 2019 KCSE Past Papers Chemistry Paper 2 2019 KCSE Past Papers Chemistry Paper 3 2019 Past Papers KCSE Chemistry Paper 1 2019 Past Papers KCSE Chemistry Paper 2 2019 Past Papers KCSE Chemistry Paper 3 2019 Chemistry Form 1 Questions and Answers Chemistry Paper 1 2018 Chemistry Paper 2 2018 Chemistry Paper 1 2019 Chemistry Paper 2 2019 Chemistry Paper 1 Notes Chemistry Paper 1 Questions and Answers Pdf Chemistry Paper 2 Questions and Answers Chemistry Questions and Answers Form 2 KCSE Chemistry Paper 1 2018 KCSE Chemistry Paper 2 2018 KCSE Chemistry Paper 1 2017 KCSE Chemistry Paper 2 2017 KCSE Chemistry Paper 1 2019 KCSE Chemistry Paper 2 2019 Most Tested KCSE Chemistry Questions 4m1 Notes Viusasa 4m2 Notes Viusasa 4m3 Notes Viusasa 4m4 Notes Viusasa Download on Viusasa - Download Now for Free Elimu - Viusasa Elimu Library | Notes, Exams, Lesson Plans, Schemes Elimu Online High School Notes - Revision Materials for Kenyan Schools Https //www.viusasa.com/elimu Kenya Notes Viusasa Elimu Form 1 Notes Viusasa Elimu Form 2 Notes Viusasa Elimu Form 3 Notes Viusasa Elimu Form 4 Notes Viusasa Elimu Form Four Notes Viusasa Elimu Form One Notes Viusasa Elimu Form Three Notes Viusasa Elimu Form Two Viusasa Viusasa Education Viusasa Elimu Viusasa Elimu Class 6 Viusasa Elimu Form 1 Viusasa Elimu Form 1 Notes Viusasa Elimu Form 2 Viusasa Elimu Form 2 Notes Viusasa Elimu Form 3 Viusasa Elimu Form 3 Notes Viusasa Elimu Form 4 Viusasa Elimu Form 4 Notes Viusasa Elimu Form Four Viusasa Elimu Form Four Notes Viusasa Elimu Form One Viusasa Elimu Form One Notes Viusasa Elimu Form Three Viusasa Elimu Form Three Notes Viusasa Elimu Form Two Viusasa Elimu Form Two Notes Viusasa High School Notes - Revision Materials for Kenyan Schools Viusasa Notes Chemistry Mock Papers Chemistry Paper 1 2019 Chemistry Paper 1 Notes Chemistry Paper 1 Questions and Answers Chemistry Paper 1 Questions and Answers Pdf Chemistry Paper 2 Form 3 Chemistry Paper 2 Notes Chemistry Paper 2 Questions and Answers Pdf Chemistry Past Papers Chemistry Past Papers Pdf Chemistry Questions and Answers Pdf Chemistry Revision Questions and Answers Pdf Chemistry Revision Questions Pdf Common Exam Questions in Chemistry Paper 1 Common Exam Questions in Chemistry Paper 2 Common Test Questions in Chemistry Paper 1 Common Tested Questions in Chemistry Paper 1 Commonly Tested Questions in Chemistry Paper 1 K.c.s.e.Chemistry Questions and Answers KCSE 2019 Chemistry Paper 1 Marking Scheme KCSE 2019 Chemistry Paper 2 KCSE 2019 Chemistry Paper 2 Marking Scheme KCSE 2020 Prediction Questions and Answers KCSE Chemistry Paper 1 2019 KCSE Chemistry Paper 2 2016 KCSE Chemistry Paper 2 2017 KCSE Chemistry Paper 2 2018 KCSE Chemistry Paper 2 2019 KCSE Chemistry Questions and Answers Most Tested Questions in Chemistry Paper 1 Most Tested Questions in Chemistry Paper 2 Mostly Tested Questions in Chemistry Paper 1 Mostly Tested Questions in Chemistry Paper 2 Chemistry Form 4 Chemistry Revision Questions Form 1 Combined Science Notes Form 1 Form 4 Notes High Flyer Series Chemistry Form 1-4 Kcse Past Papers Chemistry With Answers Chemistry Notes Download Secondary Chemistry Notes Pdf Water and Hydrogen Form 1 Notes High Flyer Series KCSE Revision in Chemistry High Flyer Series KCSE Revision Chemistry Form 1-4 Revised A Level Chemistry Notes Uganda Pdf Download Buddo Junior School Holiday Work Chemistry Notes for a Level Pdf Chemistry Notes Form 1 Chemistry Notes Form 3 the Mole Chemistry Notes Form 4 Chemistry Notes O Level Chemistry Notes O Level Uganda Chemistry Notes O Level Uganda Pdf Chemistry Notes Pdf Download Chemistry Notes Form 3 Chemistry Notes Form 3 Chemistry Questions and Answers Form Three Chemistry Syllabus Pdf Gayaza High School Chemistry Notes Gayaza High School Chemistry Notes Pdf Gayaza High School Chemistry Past Papers Gayaza High School Chemistry Past Papers Answers Gayaza High School Chemistry Past Papers Questions and Answers Gayaza High School E Learning Gayaza High School Elearning Platform Gayaza High School Examinations Gayaza High School Holiday Work Gayaza High School Notes Gayaza High School Notes 2021 Gayaza High School Notes 2022 Gayaza High School Notes 2023 Gayaza High School Notes Pdf Gayaza Junior School E Learning Platform Gayaza Junior School Holiday Work Gayaza O Level Chemistry Notes Home › Gayaza High School | Elearning Platform Kabojja Junior School Holiday Work Pdf Klb Chemistry Book 3 Klb Chemistry Form 3 Teachers Guide Klb Chemistry Notes List of Ugandan E-learning Platforms for Students Mirembe Junior School Holiday Work Ntare School Past Papers O Level Chemistry Notes Free Download O Level Chemistry Notes in Uganda O Level Chemistry Notes Pdf O Level Chemistry Notes Uganda Pdf O Level Chemistry Notes Uganda Pdf Download O Level General - Home › Gayaza High School | Elearning S.1 Chemistry Notes | Standard High School Zzana S.3 Chemistry Notes S.4 Chemistry 2 Notes | Standard High School Zzana Seeta High School Elearning Seeta High School Holiday Work Seeta High School Notes Seeta High School Notes Pdf Seeta High School Past Papers Seeta High School Past Papers Pdf Senior 1 Chemistry Notes Senior 1 Chemistry Notes in Uganda Senior 1 Chemistry Notes Uganda Senior 1 Chemistry Questions Senior 1 Exams Senior 1 Work Senior 1 Work 2020 Senior 1 Work 2020 Uganda Senior 2 Chemistry Notes Senior 2 Chemistry Notes in Uganda Senior 2 Chemistry Notes Uganda Senior 2 Chemistry Questions Senior 2 Exams Senior 2 Work Senior 2 Work 2020 Senior 2 Work 2020 Uganda Senior 3 Chemistry Notes Senior 3 Chemistry Notes in Uganda Senior 3 Chemistry Notes Uganda Senior 3 Chemistry Questions Senior 3 Exams Senior 3 Work Senior 3 Work 2020 Senior 3 Work 2020 Uganda Senior 4 Chemistry Notes Senior 4 Chemistry Notes in Uganda Senior 4 Chemistry Notes Uganda Senior 4 Chemistry Questions Senior 4 Exams Senior 4 Work Senior 4 Work 2020 Senior 4 Work 2020 Uganda Senior Four Chemistry Notes Senior Four Chemistry Notes in Uganda Senior Four Chemistry Notes Uganda Senior Four Chemistry Questions Senior Four Exams Senior Four Work Senior Four Work 2020 Senior Four Work 2020 Uganda Senior One Chemistry Notes Senior One Chemistry Notes in Uganda Senior One Chemistry Notes Uganda Senior One Chemistry Questions Senior One Exams Senior One Work Senior One Work 2020 Senior One Work 2020 Uganda Senior Three Chemistry Notes Senior Three Chemistry Notes in Uganda Senior Three Chemistry Notes Uganda Senior Three Chemistry Questions Senior Three Exams Senior Three Work Senior Three Work 2020 Senior Three Work 2020 Uganda Senior Two Chemistry Notes Senior Two Chemistry Notes in Uganda Senior Two Chemistry Notes Pdf Senior Two Chemistry Notes Uganda Senior Two Chemistry Questions Senior Two Exams Senior Two Work Senior Two Work 2020 Senior Two Work 2020 Uganda St Mary's Kitende E Learning Standard High School Notes Standard High School Zana a Level Notes Standard High School Zana Com Notes Standard High School Zana E Learning Standard High School Zana E Learning Platform Standard High School Zana E-learning Platform Standard High School Zana Notes for Senior Two Standard High School Zana Notes Pdf Standard High School Zana Website Standard High Zana Notes Uace Chemistry Notes Uace Chemistry Notes Pdf Uce Chemistry Notes Pdf Uce Chemistry Notes Pdf Download Uce Past Papers Uganda Secondary Schools E-learning Platform Uneb Marking Guides Pdf Uneb Past Papers and Answers Pdf Air and Combustion Form 1 Notes Chemistry Form 1 Notes Pdf Free Download Chemistry Form One Questions and Answers Chemistry Notes Form 1 Easy Elimu Chemistry Notes Form 1-4 Pdf Chemistry Notes Form 1-4 Pdf Download Free High School Chemistry Notes Pdf Klb Chemistry Form 1 Notes Pdf Download Notes of Form One Chemistry Laboratory and Drawings Chemistry Form 1 Questions and Answers Pdf Download Chemistry Notes Form 1-4 Chemistry Form 1 Questions and Answers Pdf Form 1 Chemistry Topical Questions Form 1 Chemistry Exam Paper With Answer Pdf Form 1 Exams 2020 Form 1 Chemistry Past Papers Form 1 Chemistry Revision Papers With Answers Sample Chemistry Test for Form One Exams Ntare School Past Papers Seeta High School Notes Pdf Seeta High School Past Papers Seeta High School Past Papers Pdf St Mary's Kitende Past Papers Uce Chemistry Notes Pdf Uce Past Papers Uneb Marking Guides Pdf Uneb Past Papers and Answers Pdf Chemistry Book 3 Download Chemistry Form 3 Questions and Answers Chemistry Form 3 Syllabus Chemistry Form 3 Topics Chemistry Notes Form 3 Chemistry Notes Form 3 Chemistry Notes Form 3 Chemistry Notes Form Three Chemistry Notes Pdf Download Form 3 Chemistry Exam Form 3 Chemistry Notes on Pdf Revision Chemistry Revision a Level Chemistry Revision Notes - Chemistry S.1-Chemistry-notes S.1-Chemistry-notes Uganda S.2-Chemistry-notes S.2-Chemistry-notes Uganda S.3-Chemistry-notes S.3-Chemistry-notes Uganda S.4-Chemistry-notes S.4-Chemistry-notes Uganda S.5-Chemistry-notes S.5-Chemistry-notes Uganda S.6-Chemistry-notes S.6-Chemistry-notes Uganda S1 Chemistry Notes Term 1 S1 Chemistry Notes Term 2 S1 Chemistry Notes Term 3 S2 Chemistry Notes Term 1 S2 Chemistry Notes Term 2 S2 Chemistry Notes Term 3 S3 Chemistry Notes Term 1 S3 Chemistry Notes Term 2 S3 Chemistry Notes Term 3 S4 Chemistry Notes Term 1 S4 Chemistry Notes Term 2 S4 Chemistry Notes Term 3 Senior 3 Chemistry Notes Senior 3 Chemistry Notes Uganda Senior Three Chemistry Notes Senior Three Chemistry Notes Uganda Chemistry Form 1 the Cell Chemistry Form One Notes Free – Education News Chemistry Notes Form 1-4 Chemistry Questions and Answers Form 1 - Chemistry Form One Chemistry Questions and Answers Form 2 - Chemistry Form Two Chemistry Questions and Answers Form 3 - Chemistry Form Three Chemistry Questions and Answers Form 4 - Chemistry Form Four Chemistry Questions and Answers Senior 1 - Chemistry Senior One Chemistry Questions and Answers Senior 2 - Chemistry Senior Two Chemistry Questions and Answers Senior 3 - Chemistry Senior Three Chemistry Questions and Answers Senior 4 - Chemistry Senior Four Chemistry Topic by Topic Questions and Answers Combined Science Notes Form 1 Form 1 Chemistry Revision Questions and Answers Form 1 Chemistry Topical Questions Form 1 Revision Papers With Answers Form 2 Chemistry Revision Questions and Answers Form 3 Chemistry Revision Questions and Answers Form Four Chemistry Revision Questions and Answers Form One Chemistry Revision Questions and Answers Form Three Chemistry Revision Questions and Answers Form Two Chemistry Revision Questions and Answers Free Chemistry Form 1 Notes Free Chemistry Notes Introduction to Chemistry Form One Kcse Past Papers: Chemistry Form 1 Kcse Past Papers: Chemistry Form 1 Topical Questions Kcse Past Papers: Chemistry Form 1 Topical Questions and Answers Most Tested Areas in Chemistry Kcse Most Tested Areas in Chemistry Kcse Exams Most Tested Areas in Chemistry Uneb Most Tested Areas in Form 1 in Chemistry Most Tested Areas in Form 2 in Chemistry Most Tested Areas in Form 3 in Chemistry Most Tested Areas in Form 4 in Chemistry Most Tested Areas in Form Four in Chemistry Most Tested Areas in Form One in Chemistry Most Tested Areas in Form Three in Chemistry Most Tested Areas in Form Two in Chemistry Most Tested Areas in Kcse Chemistry Most Tested Areas in Kcse Chemistry Exams Most Tested Areas in Senior 1 in Chemistry Most Tested Areas in Senior 2 in Chemistry Most Tested Areas in Senior 3 in Chemistry Most Tested Areas in Senior 4 in Chemistry Most Tested Areas in Senior Four in Chemistry Most Tested Areas in Senior One in Chemistry Most Tested Areas in Senior Three in Chemistry Most Tested Areas in Senior Two in Chemistry Most Tested Areas in Uneb Chemistry Revision Notes Chemistry Form 1 - Free Kcse Past Papers Who Was Chemistry Champion KCSE 2019 Top 100 Students in Chemistry KCSE 2019 Best 100 Students in Chemistry KCSE 2019 Top Student in Chemistry KCSE Best Student in Chemistry KCSE Who Was Chemistry Champion KCSE 2020 Top 100 Students in Chemistry KCSE 2020 Best 100 Students in Chemistry KCSE 2020 Names of Best Chemistry Students KCSE Names of Top Chemistry Students KCSE High School Chemistry Notes Pdf Best High School Chemistry Notes Pdf Comprehensive High School Chemistry Notes Pdf Klb Chemistry Form 1 Book Pdf Chemistry Form 1 Best Notes Chemistry Form 1 Notes Pdf Chemistry Form 1 Pressure Chemistry Form 1 Questions and Answers Chemistry Form 1 Questions and Answers Pdf Download Chemistry Form 2 Best Notes Chemistry Form 3 Best Notes Chemistry Form 4 Best Notes Chemistry Form Four Best Notes Chemistry Form One Best Notes Chemistry Form Three Best Notes Chemistry Form Two Best Notes Chemistry Notes Form 1 to 4 Chemistry Notes Pdf
Scholarship 2024/25
Current Scholarships 2024/2025 - Fully Funded
Full Undergraduate Scholarships 2024 - 2025
Fully Funded Masters Scholarships 2024 - 25
PhD Scholarships for International Students - Fully Funded!
Funding Opportunities for Journalists 2024/2025
Funding for Entrepreneurs 2024/2025
***
